Fig 2.
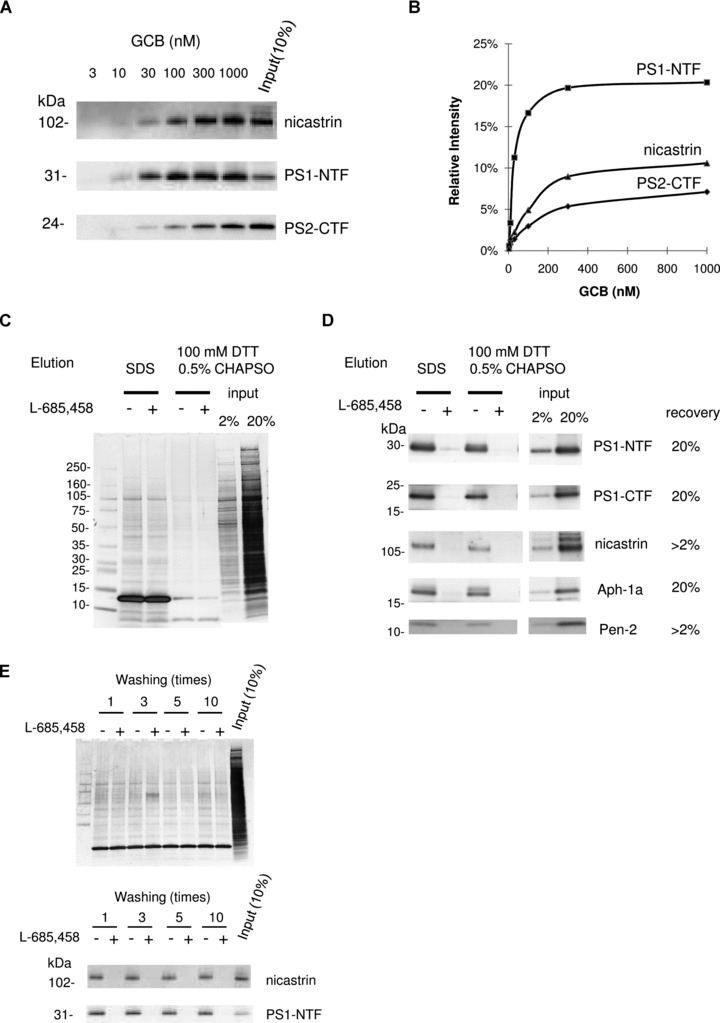
γ-secretase complex components were specifically captured by GCB pulldown from rat brain and eluted by reducing conditions. (A) Solubilized γ-secretase prepared from rat brain material was incubated with increasing concentrations of GCB and isolated with SA beads. The captured γ-secretase complex was eluted by SDS sample buffer and subject to Western blotting for the indicated γ-secretase subunit. (B) The recovery of nicastrin, PS1-NTF and PS2-CTF was estimated by quantifying the density of the respective bands and the input (10% of the total sample) by a CCD camera and appropriate software. (C) Solubilized γ-secretase prepared from rat brain material was incubated with 200 nM GCB in the presence (+) or the absence (−) of 10 μM L-685,458 and isolated with SA beads. The captured γ-secretase complex was eluted with SDS sample buffer or 100 mM DTT supplement with 0.5% CHAPSO. Eluted samples were separated by SDS-PAGE and transferred to PVDF membrane. Transferred membrane was analysed by colloidal gold staining. Elution using reducing reagent (DTT) clearly reduced non-specific binding compared to elution using SDS sample buffer. Also the elution of SA (13 kD) was reduced. (D) The same samples as in (C) were subjected to Western blotting for the indicated γ-secretase subunit. (E) Solubilized γ-secretase prepared from rat brain material was incubated with 200 nM GCB in the presence (+) or the absence (−) of 10 μM L-685,458 and isolated with SA beads. The resin was washed indicated times with buffer A with 0.5% CHAPSO at room temperature and eluted with SDS sample buffer. Eluted samples were subjected to Western blotting for the indicated γ-secretase subunit and analysed by colloidal gold staining.
