Table 1.
Fluorination and Ligands Enable Regioselective Allylation Reactions.[a]
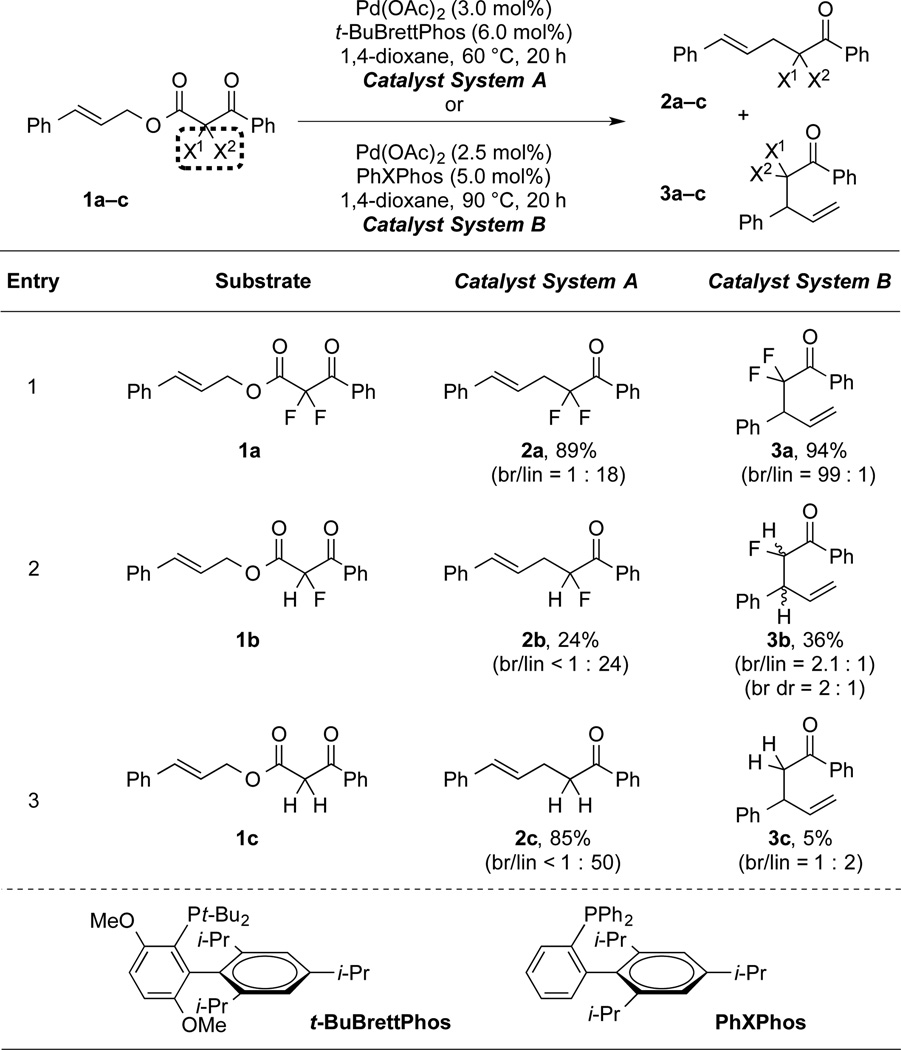 |
Catalyst System A: substrate (1.0 equiv), Pd(OAc)2 (3.0 mol%), t-BuBrettPhos (6.0 mol%), 1,4-dioxane (0.50 M), 60 °C, 20 h; Catalyst System B: substrate (1.0 equiv), Pd(OAc)2 (2.5 mol%), PhXPhos (5.0 mol%), 1,4-dioxane (0.10 M), 90 °C, 20 h. For fluorinated products, yields and selectivities were determined by 19F NMR using PhCF3 or PhF as an internal standard, respectively. For non-fluorinated products, yields and selectivities were determined by 1H NMR using CH2Br2 as an internal standard.
