Table 2.
Reactions of Substrates Bearing Distinct Allyl Moieties.[a]
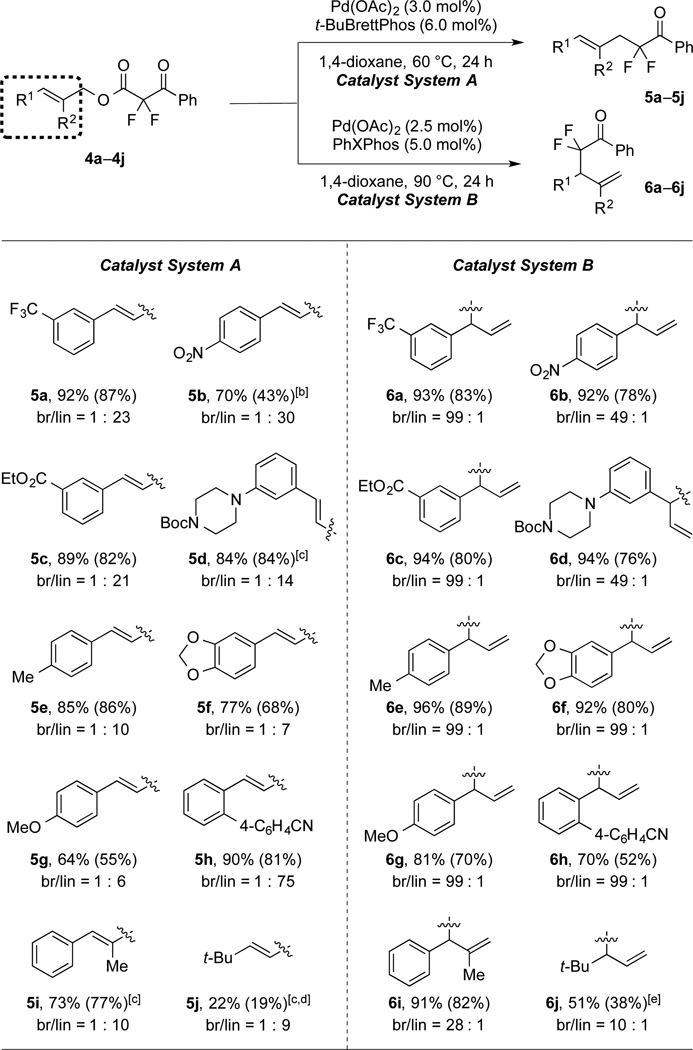 |
Catalyst System A: 4a–j (1.0 equiv), Pd(OAc)2 (3.0 mol%), t-BuBrettPhos (6.0 mol%), 1,4-dioxane (0.50 M), 60 °C, 24 h; Catalyst System B: 4a–j (1.0 equiv), Pd(OAc)2 (2.5 mol%), PhXPhos (5.0 mol%), 1,4-dioxane (0.10 M), 90 °C, 24 h. 19F NMR yields for the major isomers were determined using PhCF3 as an internal standard (average of two runs). The values in parentheses represent the yields of the major products. The regioselectivities were determined by 1H NMR analysis of the crude reaction mixtures.
70 °C.
Pd(OAc)2 (5 mol%), t-BuBrettPhos (10 mol%).
100 °C.
130 °C, o-Xylene, the regioselectivities were determined by GC and 19F NMR of the crude reaction mixtures.
