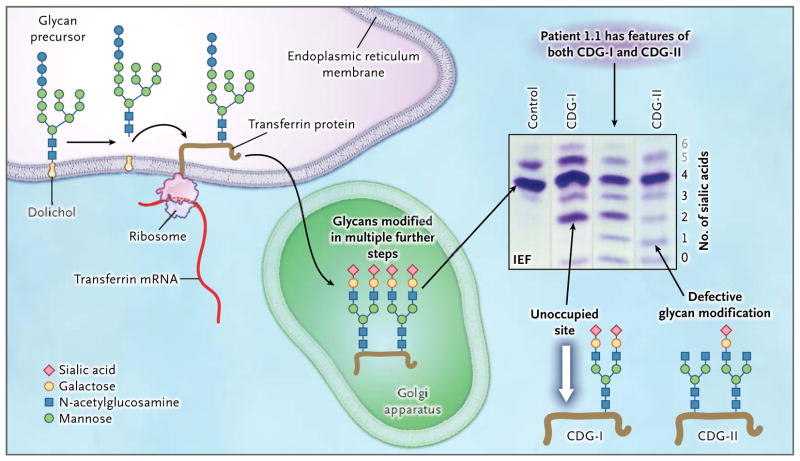
Figure 1. Glycoprotein Biosynthesis and Congenital Disorders of Glycosylation.
For the N-glycosylation of proteins, a glycan precursor is assembled from monosaccharide units on an anchoring isoprenoid alcohol, dolichol, in the membrane of the endoplasmic reticulum. Each glycan precursor is then transferred en bloc to a specific asparagine residue in the nascent peptide chain of a protein being synthesized by a ribosome and entering the endoplasmic reticulum. Because transferrin (brown line) is abundant in blood, it is used as an indicator of the efficiency of the glycosylation system. Transferrin has two glycosylation sites. The protein is transported to the Golgi apparatus, and the glycans are modified in multiple steps. Each fully modified glycan is terminated with two negatively charged sialic acid residues. Thus, the transferrin molecule carries a total of four sialic acid residues. If a genetic defect impairs synthesis of the precursor glycan in the endoplasmic reticulum, one or both glycosylation sites of transferrin may remain unoccupied (white arrow). Such defects are classified as congenital disorders of glycosylation type I (CDG-I). In congenital disorders of glycosylation type II (CDG-II), the defect occurs in the modification of the already-transferred glycan. Both glycosylation residues may carry a glycan, but those glycans may be missing terminal sialic acid residues and end with galactose, mannose, or N-acetylglu-cosamine. Isoelectric focusing (IEF), which separates molecules according to differences in their isoelectric point, is used for investigating congenital disorders of glycosylation with the use of an agarose-gel–based system, followed by immunoprecipitation in gel with an antibody to transferrin and final staining of the precipitate with Coomassie blue. Under conditions of normal glycosylation, most transferrin molecules carry four sialic acid residues (tetrasialo-transferrin) and form a single major band on IEF. A few transferrin molecules carry five or six sialic acid residues that appear as minor bands on IEF (gray numbers; not shown in the molecular-structure diagrams). CDG-I defects are easily identified owing to the occurrence of transferrin isoforms with two or no sialic acid residues detected by means of IEF. No signal for monosialotransferrin (transferrin with one sialic acid residue) is seen in such disorders. In CDG-II, the defect can result in transferrin molecules with no, one, two, three, or four sialic acid residues. A patient with a CDG-II defect typically has bands of approximately equal intensity at all positions from 0 through 3 or a smooth gradient of intensities, whereas in most cases, tetrasialotransferrin shows a more intense signal owing to residual activity. In our patients, the signal of transferrin with two sialic acid residues (disialotransferrin) may be more intense than those for either one or three residues (as in the sample from Patient 1.1). The signal with two sialic acid residues presumably arises from a mixture of transferrin molecules that have one unoccupied glycosylation site as well as transferrin molecules that are occupied at both sites by glycans but have only two terminal sialic acid residues. The patients in this study had features of both CDG-I and CDG-II, as shown by IEF, suggesting a novel disorder.