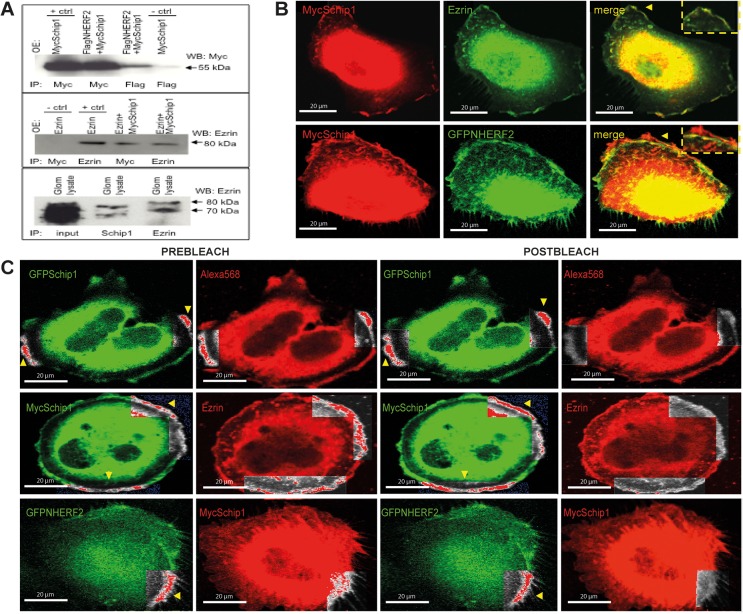
Fig 7. Schip1 interacts and colocalizes with Nherf2 and ezrin in vitro.
(A) Myc-tagged Schip1 and Flag-tagged Nherf2 coimmunoprecipitate from lysates of cotransfected HEK293 cells (upper panel). Negative control (MycSchip1 transfection, Flag IP, anti-Myc blot) is shown in lane 4 and positive control (MycSchip transfection, Myc IP, anti-Myc blot) in lane 1. MycSchip1 also interacts with ezrin, a protein known to be in complex with Nherf2, in cotransfected HEK293 (middle panel). Negative control (Ezrin transfection, Myc IP, anti-Ezrin blot) is here in lane 1 and positive control (Ezrin transfection, Ezrin IP, anti-Ezrin blot) in lane 2. Endogenous Schip1 and ezrin interact in pig glomerular lysates as shown by coimmunoprecipitation (lower panel). Whole blots are presented in S4 Fig. OE-overexpression by transfection, IP-immunoprecipitation, WB-Western blot. (B) Schip1 colocalizes with ezrin and Nherf2 in cultured human podocytes to cortical actin zones and lamellipodia. Schip1 and ezrin show very close overlap (arrowheads, boxed area, zoom), whereas Schip1 and Nherf2 colocalize partially (boxed area, zoom). (C) Interaction of Schip1 with Nherf2 and ezrin was confirmed by FRET in cotransfected, fixed podocytes. MycSchip1 signal intensity increases upon Alexa568 bleaching (boxed area, arrowheads) as a result of FRET between the two fluorophores (Alexa568 and Alexa488) suggesting associations of Schip1 with ezrin and Nherf2 proteins (middle and bottom panels). As a positive control, we used GFP-Schip1 stained with anti-GFP- and Alexa Fluor 568-conjugated secondary antibodies (upper panel). We detected about 40% FRET signal increase between Schip1 and ezrin. Lower FRET signal increase of about 10% was detected between Schip1 and Nherf2 (n = 20 ROIs tested in each experiment).