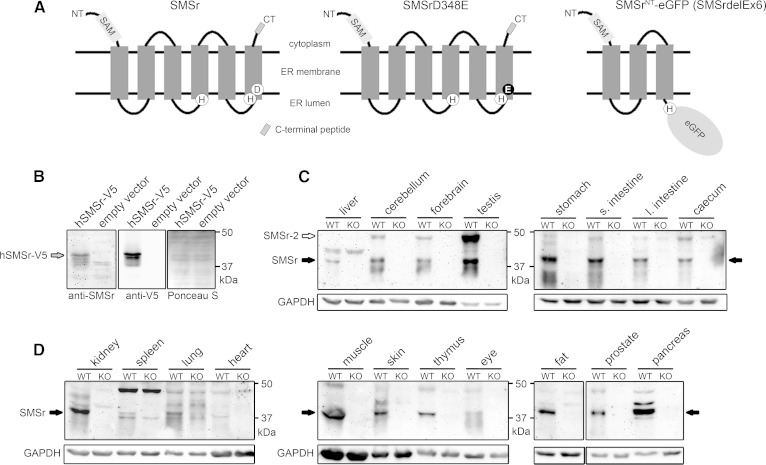
Fig. 5.
Tissue-specific expression of SMSr protein in mice. A: Predicted membrane topology of wild-type SMSr, SMSrD348E, and SMSrNT-eGFP fusion protein produced in SMSrdelEx6 mice. The eGFP reporter replaces the endogenous C terminus in the SMSrNT-eGFP (SMSrdelEx6) fusion protein and is predicted to be oriented toward the ER lumen. Lack of the C terminus allowed validating specificity of newly generated antibodies targeting the C terminus in immunoblot analysis (B–D). Sizes: Wild-type and SMSrD348E, 48.2 kDa (415 aa); SMSrNT-eGFP (SMSrdelEx6), 63.5 kDa (559 aa). B: Immunoblot analysis with lysates from yeast cells overexpressing an hSMSr-V5 vector confirmed specificity of the generated antibodies. The 49.6 kDa SMSr-V5 fusion protein was recognized by the SMSr antibodies, as well as by an antibody targeting the V5-tag. Ponceau S staining was used as loading control. C, D: Immunoblot analysis of wild-type tissues. Lysates from SMSrdelEx6 mice served as negative control and further confirmed specificity of the antibodies. Besides the 48.2 kDa SMSr protein, the antibodies recognize the predicted 54.7 kDa SMSr-2 isoform. Both isoforms did not migrate to positions expected for their theoretical mass. Using different protein standards, we show that SMSr and the corresponding SMSr-V5 are detected at about 42 kDa, whereas SMSr-2 was detected at about 48 kDa. GAPDH was used as loading control. Note: 25 μg of protein were applied for forebrain, cerebellum, and testis, 50 μg for liver and other tissues. (Data are representative of at least three independent experiments; s., small; l., large). hSMSr, human SMSr.