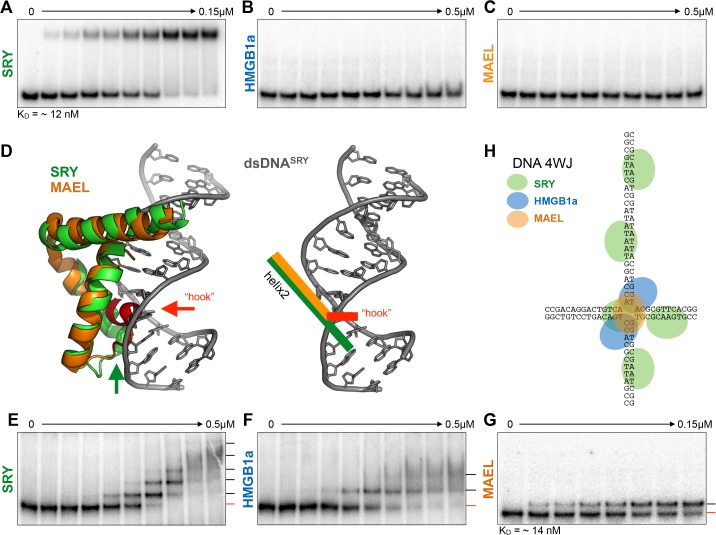
Fig 2. MAEL HMG-box domain binding to DNA.
(A) Recombinant SRY HMG-box domain strongly binds to dsDNA with its consensus sequence (dsDNASRY). (B) Recombinant HMGB1a protein does not bind to the same substrate because it is unable to bend linear dsDNA. (C) Similar to (B) MAEL HMG-box does not bind to dsDNA. (D) Representation of co-crystal structure of SRY HMG-box (green) with dsDNASRY describing their fit. Aligned with SRY HMG-box is the MAEL HMG-box domain (orange) whose “hook” region protrudes deeply into dsDNA (1j46, 2cto). The geometry of the helix-2 of both molecules in relation to dsDNASRY is highlighted on the right. Even though dsDNASRY is pre-bent, it cannot accommodate the “hook” region of MAEL HMG-box domain in the same fashion. (E) SRY HMG-box domain binds to DNA 4WJ forming five complexes (red lines—free substrate; black line—bound substrate). (F) HMGB1a binds to DNA 4WJ forming two complexes. (G) The MAEL HMG-box domain forms only single complex with DNA 4WJ, which is different from the SRY and HMGB1a domains. (H) Proposed mode of HMG-box domain binding to DNA 4WJ. SRY HMG-box domain recognizes a distorted 4WJ center as well any sequences that approximate its consensus-binding site, while HMGB1a protein binds primarily to irregular center. Just as HMGB1a, MAEL HMG-boxes does not bind dsDNA suggesting that it also binds to the center of the junction. Because only single complex forms binding likely happens in different fashion.