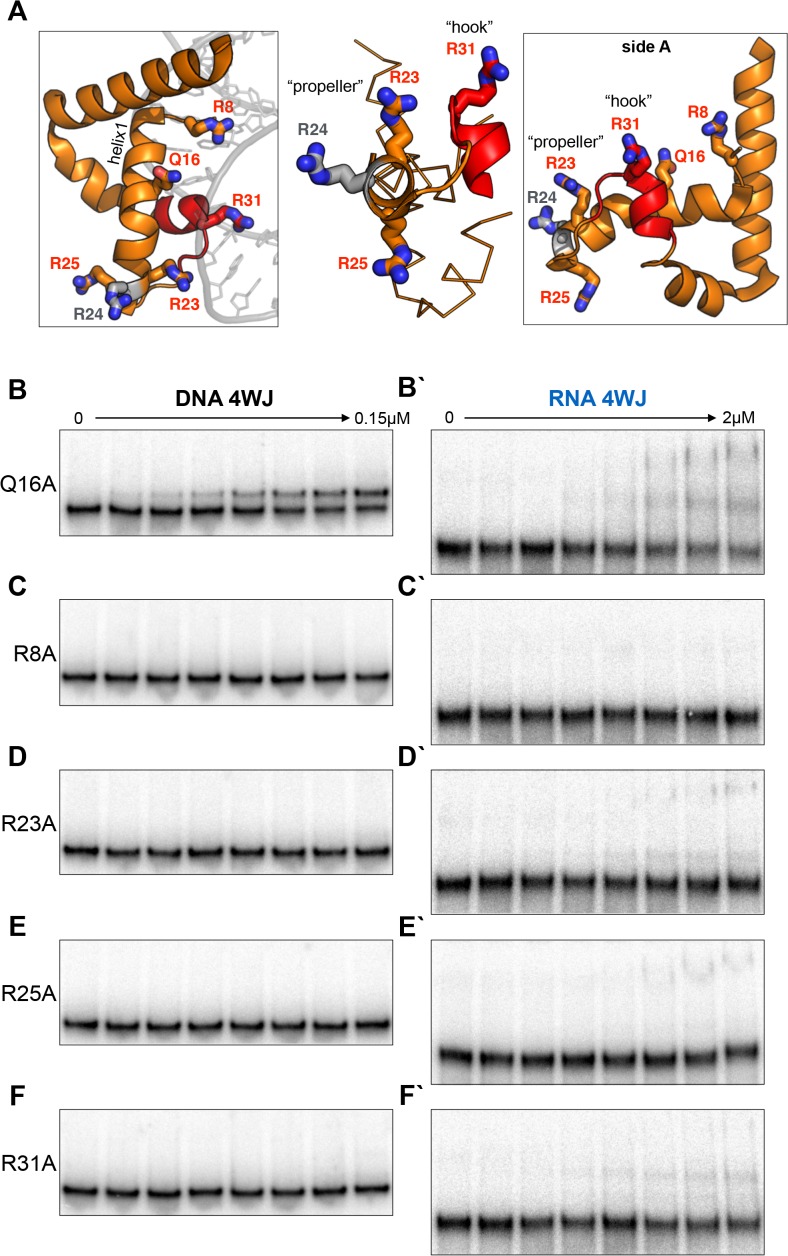
Fig 4. Site-specific mutagenesis of MAEL HMG-box domain.
(A) Charged residues, capable of H-bond formation with long side chains, along helix-1 (region important for canonical HMG-box binding) and novel regions of MAEL HMG-box domain were mutated to alanine (residues in red). (B) Mutation of glutamine (Q16A) does not affect binding suggesting that this residue does not participate in complex formation with DNA 4WJ. (C-F) Mutation of individual arginines (R) results in the complete loss of binding to DNA 4WJ suggesting that they are essential for complex formation. The R8A mutant fold appears to be affected (S2B Fig.), which might be responsible for reduced binding. Therefore, only arginines in the “hook” and “propeller” seem to be necessary for MAEL HMG-box domain binding to non-canonical DNA. (B`-F`) Interactions of mutants with RNA 4WJ are significantly affected. All mutants show greatly decreased binding to RNA 4WJ as well as variability in the type of complex formed when compared to wild-type protein (Fig. 3H). Residual complex formation was still observed (except for R8A) likely due to a high number of configurations that RNA 4WJ can take to accommodate the MAEL HMG-box domain. Nevertheless, the arginines in the “hook” and “propeller” of MAEL HMG-box are important for binding to structured RNA.