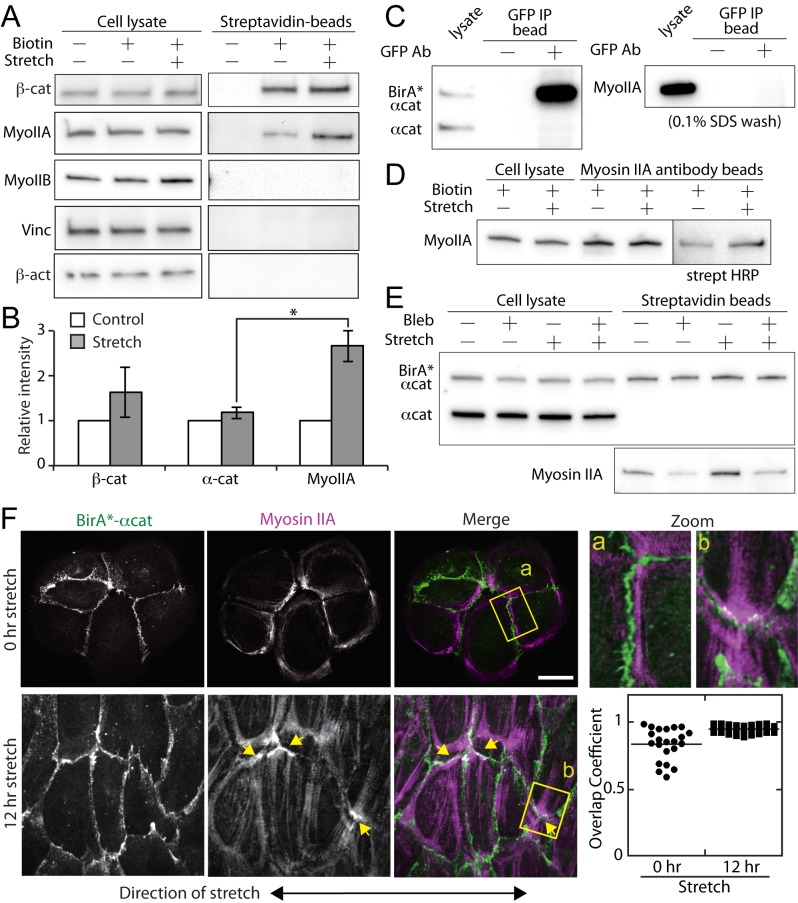
Fig 4. Increased biotinylation of myosin IIA in stretched cells.
(A) Western blot analysis of cell lysates and purified streptavidin-conjugated beads from control and stretched cells. With 0.1% SDS wash solution, the purified proteins included β-catenin and myosin IIA. The biotinlyation of myosin IIA increased in mechanically stretched samples. Myosin IIB (MyoIIB), vinculin (Vinc) and β-actin (β-act) were not purified with streptavidin-conjugated beads in unstretched or stretched samples. (B) Quantification of the relative band intensities of unstretched (control) and stretched (stretch) samples. βcat (n = 4), αcat (n = 4) and MyoIIA (n = 7). Results were analyzed using a one-way ANOVA; significance was determined using Dunnett’s post hoc test. Results were considered significant with P<0.05. (C) Immuno-precipitation of GFP-tagged BirA*-α-catenin using a GFP antibody. The immuno-precipitated samples were analyzed with α-catenin and myosin IIA antibodies. The blot of α-catenin is reproduced from Fig 2B. (D) Immuno-precipitation of myosin IIA using a myosin IIA antibody. Immuno-precipitated samples were analyzed with a myosin IIA antibody and streptavidin. (E) Blebbistatin decreases biotinlyation of myosin IIA in both unstretched and stretched samples, suggesting that myosin IIA activity is required for the spatial proximity of α-catenin and myosin IIA. (F) Post-stretching, the cells were fixed and visualized with GFP (BirA*-α-catenin) and anti-myosin IIA antibodies. Yellow arrows point to the end of actin bundles localized at cell-cell contacts where myosin IIA also accumulated. The regions denoted by yellow rectangles (a, b) are magnified in the adjacent images. See also S1 Movie for a 3D stack of stretched cells. Quantification of co-localization between BirA*-α-catenin and myosin IIA was analyzed by calculating overlapping coefficients. Scale bar 20 μm.