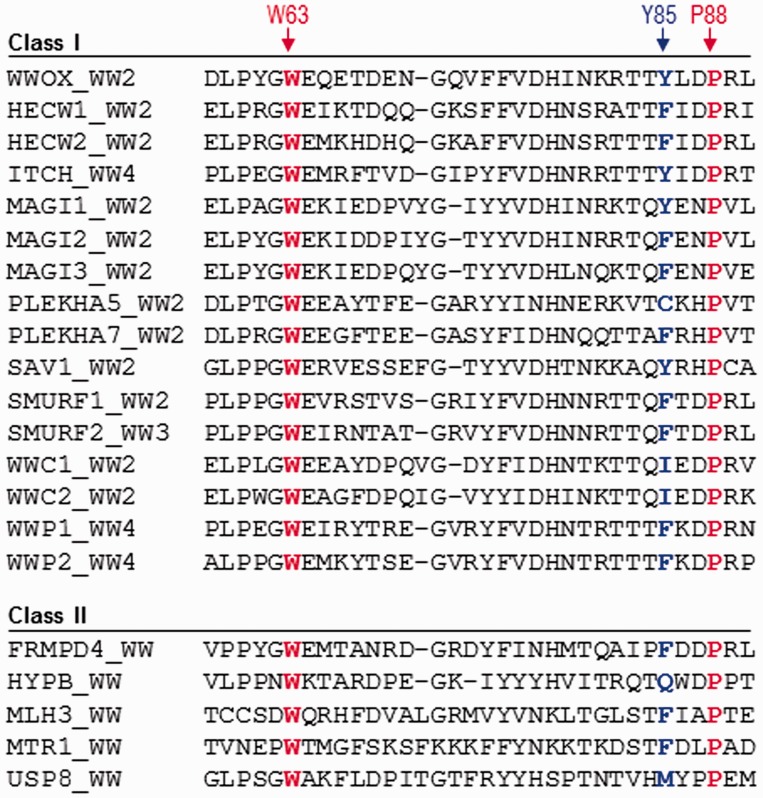
Figure 5.
Amino acid sequence alignment of WW domains within the human proteome containing a non-tryptophan residue at the structurally equivalent position occupied by Y85 within the WW2 domain of human WWOX. Note that the sequence alignment is subdivided depending on whether the WW domains belong to multi-copy-WW-containing proteins (Class I) or single-copy-WW-containing proteins (Class II). The Y85 residue within the WW2 domain of WWOX and its structural equivalents within other WW domains are colored blue. Absolutely conserved tryptophan (W63) and proline (P88), which together represent a pair of residues critical for the folding of WW domains, are shown in red. It is noteworthy that the indole and pyrrolidine side chain rings of these conserved residues engage in stacking interactions on the convex face of WW domains, right beneath or underside of the concave ligand binding groove. In so doing, they provide a critical scaffold that is essential for the structural integrity of all WW domains. (A color version of this figure is available in the online journal)