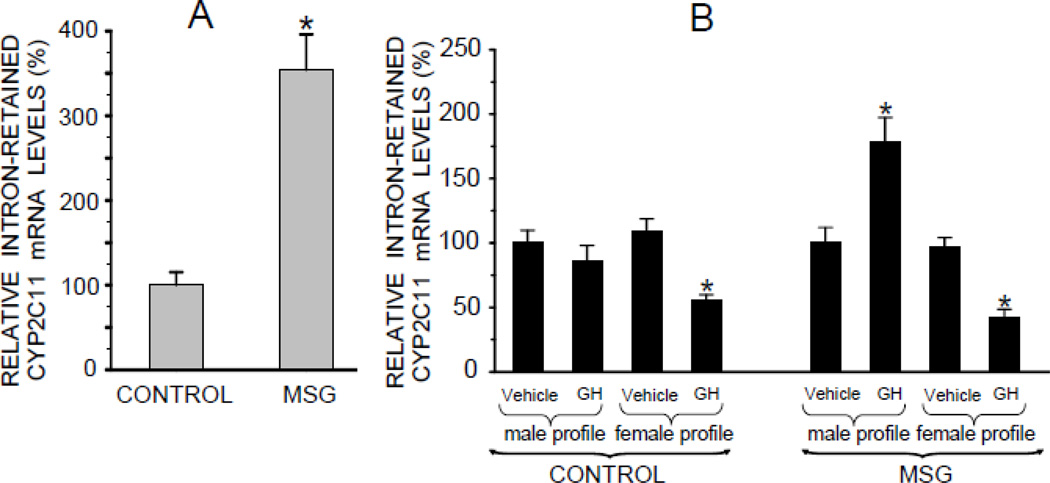
Fig. 3.
Relative mRNA expression levels of an intron-retained male-specific CYP2C11 isoform in hepatocytes from adult male rats that were either neonatally administered MSG or vehicle. Neonatally-treated pups were injected with either MSG (2mg/g bd wt) or equivalent doses of vehicle (Control) on postnatal days 1, 3, 5, 7 and 9. A. Intron-retained CYP2C11 levels in freshly isolated preplated hepatocytes. Results are presented as a percentage of mRNA in hepatocytes from control rats arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs control rats. B. Hepatocytes from the same animal were exposed to either the episodic male-like GH profile or the hormone’s vehicle, or the continuous female-like GH profile or vehicle for 6 days. Results are presented as a percentage of mRNA in hepatocytes exposed to vehicle administered in an episodic male-like profile, arbitrarily designated 100%. Values are means ±SD of at least 7 rats/data point. *P<0.01 vs hepatocytes exposed to the episodic male-like vehicle profile from rats of the same neonatal treatment, i.e. Control or MSG.