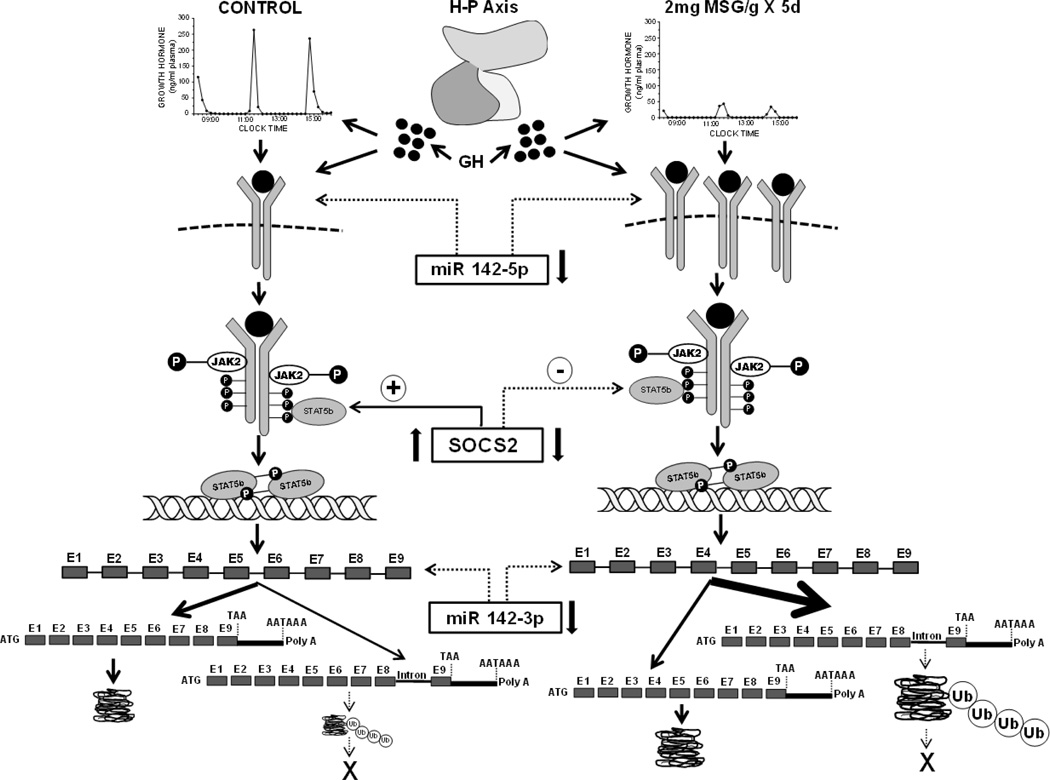
Fig. 9.
Hypothetical mechanism(s) describing the permanent effect of neonatal “low” dose MSG treatment on adult male CYP211 expression. Neonatal administration of 2mg MSG/g bd wt disrupts the development of the hypothalamic-pituitary (H-P) axis resulting in the adult secretion of mini-GH pulses in an otherwise normal masculine GH profile contributing to the permanent overexpression of CYP2C11. The mini GH secretory profile, alone, and/or in combination with reduced hepatic concentrations of miR142-5p, a putative small, single-stranded non-coding RNA suppressor of GHR transcription, results in the overexpression of the transmembrane hepatic GHR. The overexpressed GHR along with decreased levels of SOCS2, an inhibitor of GH activation of the GHR●JAK2●STAT5b complex, causes an enhanced expression of CYP2C11. This overexpression of CYP2C11 may be supported by the reduced concentrations of hepatic miR142-3p, a putative suppressor of CYP2C11 transcription. In contrast to the normal (CONTROL) condition (left side of schematic) where only a small fraction of the CYP2C11 transcript retains the terminal intron, the bulk of the CYP2C11 transcript (noted by arrow thickness) retains the intron separating exons 8 and 9. Although not studied in this report, the dramatic overexpression of the intron-retained transcript may be due, at least in part, to a reduction in nuclear splicing capacity in the affected rats (Pampori and Shapiro, 2000). The absence of a detectable translation product from the intron-retained CYP2C11 mRNA in both treatment groups (depicted by X) is likely a result of the presence of a premature stop codon leading to the immediate degradation of a likely truncated protein by ubiquitination; the catabolic enzyme complex greatly overexpressed in the MSG-treated male rats. The 40 to 50% overexpression of CYP2C11 protein in the MSG rats is likely due to a similar percent elevation in normal transcript levels.