Figure 1. Epigenetic switches controlling the GC phenotype.
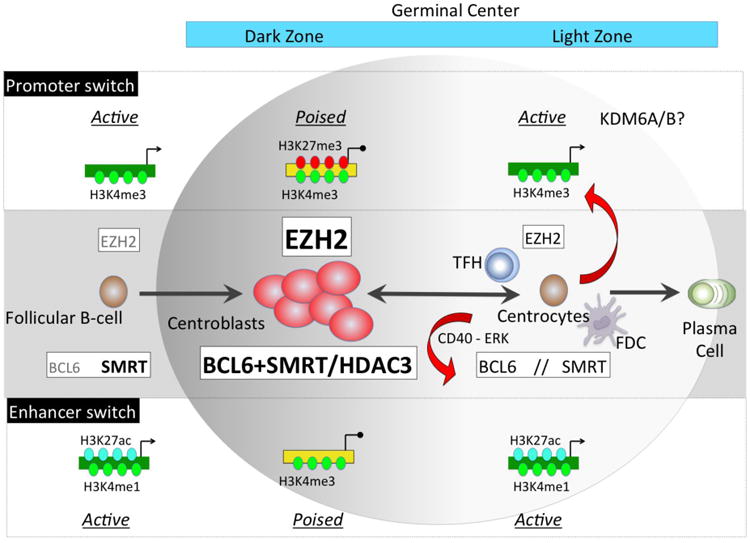
Upon T-cell dependent antigen stimulation a subset of B-cells migrate within lymphoid follicles and upregulate EZH2 and BCL6 proteins. EZH2 catalyzes addition of H3k27me3 to a set of activated promoters causing their transient transcriptional poising. BCL6 forms a complex with SMRT at active enhancers (marked with H3K4me1) causing their H3K27 deacetylation through HDAC3 and placing them in a repressed/poised configuration. Both of these switches result in transcriptional repression of genes involved in proliferation checkpoint, differentiation and apoptosis, thus defining the centroblast phenotype. When GC B-cells migrate to the GC light zone a second switch occurs via signaling from T follicular helper (TFH) and follicular dendritic cells (FDC). These result in downregulation of EZH2 and cytoplasmatic shuttling of SMRT complexes due to ERK mediated phosphorylation (red arrows). As a consequence there is histone acetylation and derepression of BCL6 targets, and H3K27 demethylation of EZH2 target bivalent promoters possibly by KDM6A/B. These events result in activation of proliferation checkpoints and induction of GC exit genes. The effects are reversible and can be switched back off in B-cells that undergo additional rounds of somatic hypermutation.
