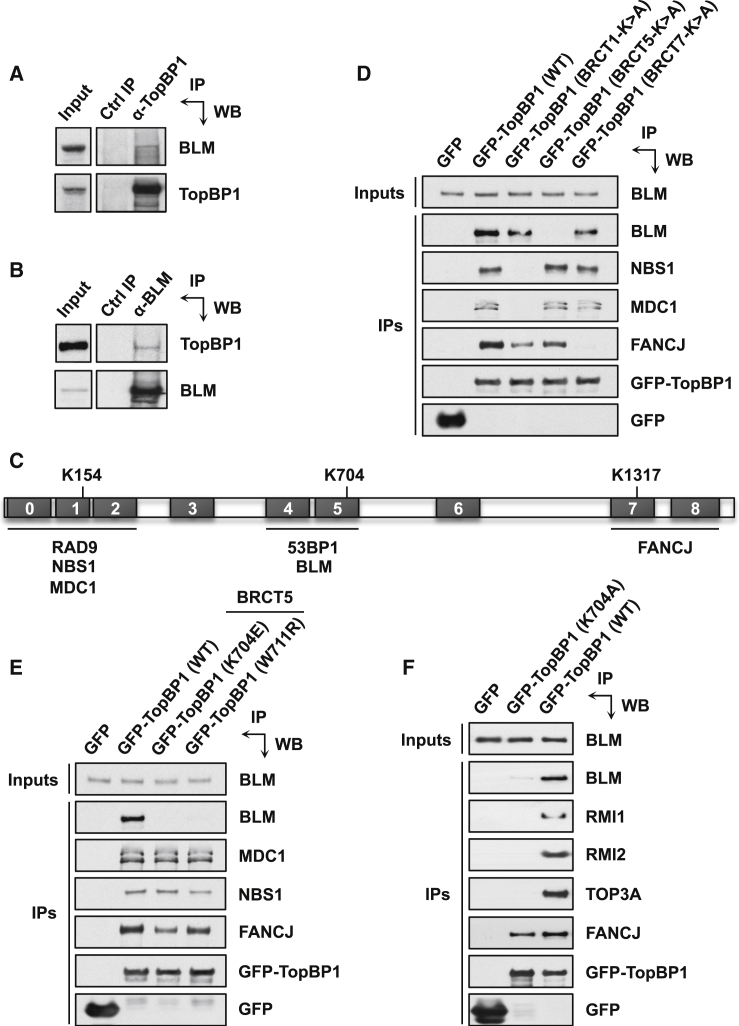
Figure 1.
BLM Interacts with TopBP1 via BRCT5
(A) TopBP1 immunoprecipitates from 293FT cell extracts contain BLM.
(B) BLM immunoprecipitates from 293FT cell extracts contain TopBP1.
(C) Schematic showing TopBP1 BRCT domain layout. Black numbered boxes represent BRCT domains. K154, K704, and K1317 are the key phosphopeptide binding lysines in BRCT domains 1, 5, and 7, respectively. The names of known TopBP1-binding partners are shown below the BRCT domains they interact with.
(D) Effect of point mutations in TopBP1 BRCT domains 1, 5, and 7 on its binding to NBS1, MDC1, FANCJ, and BLM compared to wild-type (WT). Pull-downs were carried out from 293FT cells transiently transfected with the indicated plasmids 24 hr later.
(E) Effect of two different point mutations in TopBP1-BRCT5 on binding to MDC1 and BLM. NBS1 and FANCJ are positive controls as they bind to TopBP1 BRCT domains 1 and 7, respectively.
(F) Mutation of TopBP1-BRCT5 abrogates binding to Bloom syndrome complex members. See also Figure S1.