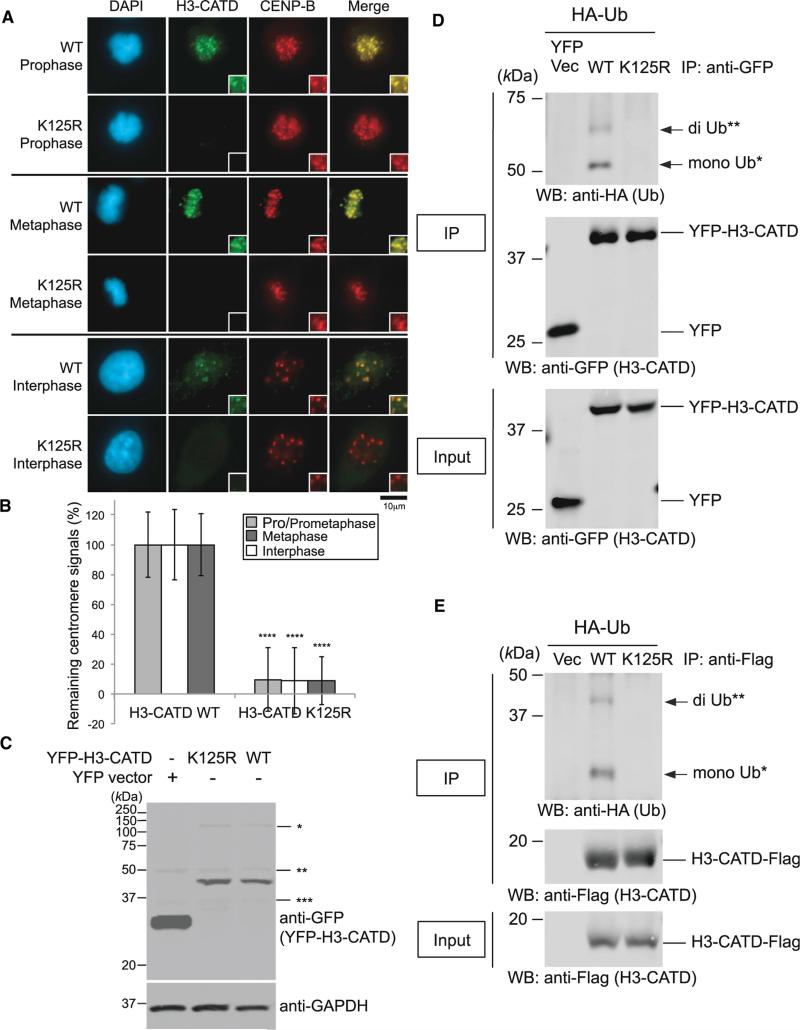
Figure 7. K125R Mutation Abrogates H3-CATD Ubiquitylation and Localization at Centromeres.
(A) Delocalization of the H3-CATD K125R mutant at centromeres. HeLa cells were cultured, transfected, and immunostained (see Immunofluorescence in Supplemental Experimental Procedures). Scale bar, 10 μm.
(B) Histogram quantifying H3-CATD WT and K125R signals at centromeres given in (A). Signals were normalized with those in H3-CATD WT, and mean percentages (±SD) are shown. ****p < 0.0001 compared with H3-CATD WT (Student's t test).
(C) Overexpressed pEYFP-H3-CATD WT and K125R were confirmed, but levels of EYFP-H3-CATD remained unchanged between the WT and K125R mutant. *, **, and *** indicate non-specific bands.
(D) The H3-CATD K125R mutation abrogates ubiquitylation of CENP-A in vivo (pEYFP vector background; see also Experimental Procedures). Proteins in 5% of the total cell lysates (Input) and immunoprecipitates (IP) were separated by a 10% SDS-PAGE. YFP Vec, pEYFP vector only; WT, pEYFP-H3-CATD WT; K125R, pEYFP-H3-CATD K125R. Bands of putative di-Ub-YFP-H3-CATD (**) and putative mono-Ub-YFP-H3-CATD (*) are indicated with arrows.
(E) The H3-CATD K125R mutation abrogates ubiquitylation of CENP-A in vivo (pTRM4 vector background; see also Experimental Procedures). Proteins in 5% of the total cell lysates (Input) and immunoprecipitates (IP) obtained using ANTI-FLAG M2 Affinity Gel (SIGMA-ALDRICH) were detected. Vec, pTRM4 vector only; WT, pTRM4-H3-CATD WT-Flag; K125R, pTRM4-H3-CATD K125R-Flag. Bands of putative di-Ub-H3-CATD-Flag (**) and putative mono-Ub-H3-CATD-Flag (*) are indicated with arrows.