Abstract
Type 2 diabetes mellitus (T2D) is a growing healthcare burden primarily due to long-term complications. Strict glycemic control helps in preventing complications, and early introduction of insulin may be more cost-effective than maintaining patients on multiple oral agents. This is an expert opinion review based on English peer-reviewed articles (2000–2012) to discuss the health economic consequences of T2D treatment intensification. T2D costs are driven by inpatient care for treatment of diabetes complications (40%–60% of total cost), with drug therapy for glycemic control representing 18% of the total cost. Insulin therapy provides the most improved glycemic control and reduction of complications, although hypoglycemia and weight gain may occur. Early treatment intensification with insulin analogs in patients with poor glycemic control appears to be cost-effective and improves clinical outcomes.
Keywords: type 2 diabetes (clinical domain), hyperglycemia, hypoglycemia, insulin, oral antidiabetic agents, healthcare economics (operational domain), cost-effectiveness
KEY MESSAGES.
Type 2 diabetes mellitus is a growing burden on healthcare services.
Despite the high cost of drug therapy versus diet and lifestyle interventions, treatment intensification with insulin analog therapy is a cost-effective strategy for improving clinical outcomes in patients with poor glycemic control.
Introduction
Worldwide, more than 284 million people have diabetes, and this number is expected to reach 439 million by 2030.1 Approximately 90% of these people have type 2 diabetes (T2D),2 leading to an increasing economic burden upon healthcare systems. Although prevention of T2D is the ideal solution and has been shown to be cost-effective in modeling studies,3 providing optimal cost-effective treatment to those with T2D is an urgent medical need.4,5 Uncontrolled blood glucose leads to microvascular complications and increases the risk of macrovascular complications.6–8 These complications have an adverse impact on quality of life (QoL), and their management is a major source of expenditure in people with T2D.7,8 Strict glycemic control is required to prevent or delay these complications, thus promoting long-term health and reduced treatment costs.
Glycemic control in T2D is managed initially by diet and lifestyle interventions, followed by use of oral antidiabetic drugs (OADs) and incretin-based therapies. These therapies and their associated costs have been comprehensively reviewed.9–11 Historically, insulin-based therapy has been used as a ‘last resort’ in patients with T2D; however, the benefits of earlier initiation of insulin are now generally recognized,12 including improved glycemic control and reductions in diabetes complications.9–11
The aims of this narrative review are to highlight the importance of health economic (HE) evaluations of T2D treatments in Europe, examine select HE studies in patients with T2D, and provide an assessment of this literature with respect to the HE consequences of treatment intensification. In particular, because questions remain unanswered concerning the best strategies for initiating and managing T2D with insulin therapies and their overall impact on the costs of treatment, we will focus this review on the economic implications of insulin-based therapies in T2D.
Methods
Articles for consideration for this expert opinion review were identified using a PubMed search restricted to English language publications from 2000 to 2012, using ‘type 2 diabetes mellitus’ or ‘insulin’ (title term) and ‘economics’ (MeSH term). Search outputs were further limited to peer-reviewed articles and those pertaining to EU countries. Following this search, inclusion of data in this article was determined subjectively by the authors based on the relevance to the English-speaking EU prescriber.
Results
Cost of T2D management
Landmark European studies have shown that treatment of T2D is very costly.5,7,8,13–16 For example, in the T2D Accounting for a Major Resource Demand in Society (T2ARDIS; n = 1578) survey in the UK, the average annual National Health Service (NHS) cost per patient in 2000 was £1738 (€2639),a driven primarily by the cost of hospital care (Fig. 1).7 In this study, patients visited their general practitioners an average of five times a year. Similarly, the Cost of Diabetes Type II in Europe (CODE-2) study, conducted in eight European countries, reported the total annual direct medical costs associated with T2D to be €29 billion (1999 values).13
Figure 1.
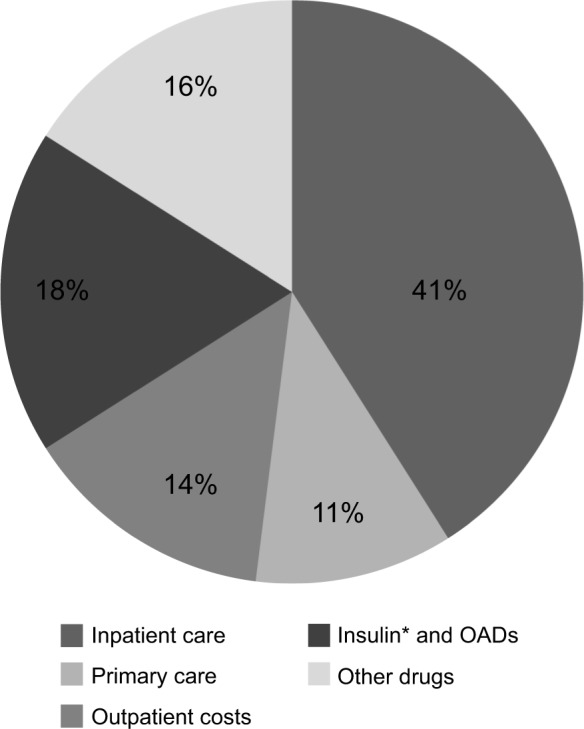
Distribution of T2D-related annual costs (UK).
Notes: Percent of patients with various complications: microvascular, 24% (eye problems, kidney damage, amputation, foot or leg ulcer); macrovascular, 12% (stroke, heart attack); none, 57%. *Includes delivery systems (pens, cartridges). Figure produced with data from Bottomley JM. Br J Diabetes Vasc Dis. 2001 (T2ARDIS study).
A study commissioned by Diabetes UK reported that, during the decade from 1997 to 2007, the mean prescribing costs for T2D patients increased by 89% (from £391 to £740 prescribing costs per person per year [pppy]) and the total costs of primary care rose by 79% (from £602 to £1080 pppy).4 For perspective, over the same period, the rate of inflation in the UK was approximately 28%.17 The increase in diabetes costs was partially due to a doubling in the number of general practitioner consultations (including surgery, home, community clinic visits, and telephone consultations) from 5.4 pppy in 1997 to 11.5 pppy in 2007. Despite increased expenditure, glycemic control did not improve over the same period; however, improvements in blood pressure and lipids were noted.4 By 2010/2011, the total cost of T2D in the UK was estimated to be £8.8 billion.18
Cost of T2D complications
Diabetes complications are an important cost driver in the overall cost of T2D mana gement.6–8,19 In the T2ARDIS survey, the presence of complications increased the primary care costs 5.6-fold, with microvascular complications leading to a 2.5-fold increase.7 In the CODE-2 study, 24% of patients had both micro- and macrovascular complications, resulting in a total cost increase of 250% compared with patients who had no complications.8 For a Spanish population within the CODE-2 study, the presence of both micro- and macrovascular complications increased the mean cost per patient by 142%.20
Diabetic drug cost is small compared to the cost of managing T2D complications; for example, in the T2ARDIS survey, only 18% of total cost was for insulin and OADs, while almost the same amount (16%) was spent on nondiabetic drugs (largely for treating macrovascular complications) (Fig. 1).7 More appropriate and effective use of diabetes drugs might therefore reduce total drug expenditure and other costs associated with the management of long-term complications. The majority of the increased cost associated with T2D complications results from longer and more frequent hospital admissions.14
Impact of treatment intensification on cost of T2D
Given that the high costs of managing T2D are driven in large measure by complications that are a consequence of poor glycemic control, the goal for T2D patients is to attain and maintain glycemic control. Research shows that intensive blood glucose control can reduce the risk of diabetes complications and the cost of managing these complications over periods from 10 years to a lifetime.21–24 As part of the UK Prospective Diabetes Study (UKPDS), intensive blood glucose control was seen to increase treatment costs by £695 (€1055)a per patient with T2D, but reduced the cost of complications by £957 (€1453)a compared to conventional management over a mean 10-year follow-up.24 Also, intensive blood glucose control produced an incremental cost per quality-adjusted life year (QALY) gain of £6028 (€8885)a.22 While conclusions on the cost-effectiveness of a therapy depend on many factors, one widely used threshold (as used by the National Institute for Health and Care Excellence [NICE]) classifies treatments as cost-effective if their incremental cost per QALY gained is less than £20,000–30,000.25 Options for intensification of blood glucose control treatment include education and self-management, combinations of OADs, incretin therapies, and insulin.
Education and self-management
The cost-effectiveness of diabetes self-management training and patient medical and nutritional education has received much attention; however, studies assessing the impact of diabetes education have reported mixed results in terms of impact on costs and patient outcome.26–29
The most robust study to be performed to date in newly diagnosed T2D patients was the Diabetes Education and Self-Management for Ongoing and Newly Diagnosed (DESMOND) program, which showed that a six-hour group educational program on self-management was cost-effective at one year follow-up compared with usual care, with particular benefit for reductions in weight, smoking, and depression,27,30 as well as improvement in self-efficacy that was sustained at three-year follow-up.31 However, a systematic review of T2D patient education models showed mixed results in terms of metabolic control and no clear characterization of which educational features may be beneficial.29 A further review of studies, comparing individual education with usual care or group education in T2D, suggested a benefit of individual education on glycemic control when compared with usual care in a subgroup of those with baseline glycated hemoglobin (A1C) >8%, but no benefit in the general T2D population and no advantage over group education.28
Guideline-specified A1C targets, which are based on optimizing clinical outcomes, may be unrealistic for some patients; patient management may be better focused on healthy lifestyle, preventive care, and reducing cardiovascular risk with glycemic control tailored to individual patient circumstances.32 The benefits of tight glycemic control are best realized when a patient is proficient at regularly self-monitoring blood glucose (SMBG). SMBG provides immediate feedback on the impact of food choices, exercise, and medication on glycemia, and helps avoid hypoglycemic events.33 It is generally recommended that patients perform SMBG at least once a day (3–4 times for insulin therapy), varying between fasting, pre-, and postprandial times over a week.
However, a systematic review by the Aberdeen Health Technology Assessment Group (UK) regarding the value of SMBG found that it had limited clinical effectiveness for improving glycemic control in patients with T2D receiving OADs and that it was unlikely to be cost-effective in this situation.34 Therefore, frequent SMBG may be clinically necessary and cost-effective only in patients receiving insulin. Further research into the benefits of individual versus group educational activities and the merits of SMBG is warranted.
Insulin-based treatment regimens in T2D
A common guidance-based approach to T2D management is to first attempt glycemic control with diet and lifestyle changes in conjunction with metformin.35 Traditionally, various OADs are added sequentially to the regimen, even though there is limited evidence that using three or more OADs provides additional therapeutic benefit.10,36,37 In addition, hypoglycemia and weight gain are common adverse events with older agents.38 Weight gain in particular is strongly associated with increased risk of cardiovascular morbidity and increased costs.39–41 With the introduction of glucagon-like peptide-1 (GLP-1) analogs and sodium/glucose cotransporter 2 (SGLT2) inhibitors, it is now possible to improve glycemic control and potentially reduce weight when metformin alone is no longer sufficient.42 A more complete discussion of the cost-effectiveness of combining OADs, incretin therapies, and SGLT2 inhibitors is beyond the scope of this review.43
The reduction in severity and/or delayed onset of diabetes complications after achieving more effective blood glucose control using insulin therapy may be cost-effective and result in improved patient QoL.12,24,44–46 Using the IMS-CORE Diabetes Model applied to data from the UKPDS study, it was estimated that initiating insulin in patients with poor glycemic control immediately versus a delay of eight years would result in a gain of 0.61 years of life expectancy and 0.34 QALYs. These benefits were directly attributable to a delay in onset and reduced cumulative incidence of diabetes complications.47 An observational German study showed that the total average cost of diabetes care for six months following initiation of insulin rose from €579 to €961, which included costs of blood glucose monitoring and specialist care in addition to the insulin itself.48 These costs increased significantly more in patients with higher body mass index and A1C, suggesting that delay in insulin initiation may lessen its cost benefits.
Unfortunately, insulin initiation often occurs after prolonged periods of poor control,36,49 and a large proportion of patients with T2D using insulin remain poorly controlled.50,51 Insulin regimens can reduce complications and increase QoL and survival,52 but place greater demands on patients and physicians to adjust doses and increase the intensity of blood glucose monitoring.53 The use of pen injection devices for insulin delivery has been shown to improve compliance and cost-effectiveness compared to vials and syringes.54 Physicians can also influence compliance with insulin treatment regimens by being positive in their attitudes toward insulin therapy and its benefits.55
Recent HE studies have included the assessments of, and comparisons between, a number of insulin therapies, including insulins glargine and detemir (long-acting), insulin aspart (short-acting), biphasic (mixed) insulin, neutral protamine Hagedorn (NPH) insulin (intermediate-acting), and human soluble insulin. The use of insulin analogs has been shown to be more cost-effective compared to human insulin (despite higher drug costs) due to improved glycemic control and reduced propensity for hypoglycemia and weight gain.56 Differences in cost-effectiveness between the available insulin analogs depend largely on the frequency of hypoglycemia and its associated costs, although a lack of direct drug comparisons makes economic analysis difficult.9 In lieu of clinical studies, modeling data have been published. Using a computer simulation model based on a subpopulation of the observational study PREDICTIVE, a German group modeled the long-term cost-effectiveness of conversion to insulin detemir, with or without OADs, in patients failing OADs alone or in combination with NPH insulin or insulin glargine. Conversion to insulin detemir was associated with improvements in life expectancy, quality-adjusted life expectancy and cost savings, an 80% reduction in hypoglycemia rates, and a mean weight loss of 0.9 kg.44
Choice of treatment may have an influence on the occurrence of hypoglycemic events and thus on the costs of diabetes management.9 Initiation of either NPH insulin or glargine has been associated with major cost reductions (compared to an insulin-free period) and infrequent hypoglycemia-related claims.57 A meta-analysis of published literature noted that both insulin glargine and insulin detemir were associated with a lower frequency of hypoglycemia than NPH insulin, especially of nocturnal hypoglycemia.9
NICE guidelines for England and Wales58 state that in the treatment of T2D, a long-acting basal analog (insulin detemir or insulin glargine) should be considered in certain specific clinical scenarios (eg, patients unable to use NPH insulin devices or patients with hypoglycemia that restricts their lifestyle or precludes their reaching glycemic targets). NICE acknowledges that the cost-effectiveness models employed to assess insulin therapies may fail to adequately capture the impact of weight changes, fear, and other consequences of hypoglycemia, as well as other important complications (such as neuropathy), on health-related QoL.58 Thus, there is a need to continue to improve HE models to establish when it becomes cost-effective to switch from NPH to a long-acting analog and to develop models that will assist cost-conscious decision making in particular patient subgroups.
Treatment-related adverse events, such as hypoglycemia59–61 and weight gain,62 can also be associated with a significant financial burden to healthcare systems. Weight gain is associated with decreased patient utility and QoL.63 Weight loss is associated with improvements in cardiovascular risk and glycemic control in T2D but is often difficult for overweight and obese patients to achieve.
Hypoglycemic events can also have a large impact on patient QoL, and fear of hypoglycemia is a barrier to treatment compliance, leaving patients uncontrolled and at risk of complications.9,64–66 Unsurprisingly, hypoglycemic events also have an economic cost. A study considering the costs of severe hypoglycemic events in Spain, Germany, and the UK reported higher treatment costs for patients with T2D than for patients with type 1 diabetes, with average costs of €533 versus €441 in Germany, €691 versus €577 in Spain, and €537 versus €236 in the UK.67 A separate year-long study in the UK measured 244 episodes of severe hypoglycemia requiring emergency treatment in 160 patients, costing a total of £92,078 (€137,442)a.68 In Sweden, the annual cost of hypoglycemia in patients with T2D was estimated at €4,250,000 in total, equating to €14 per patient.59 There is also emerging evidence that nonsevere nocturnal hypoglycemic events may have a considerable and underestimated economic impact, for example, due to work absenteeism and loss of productivity.69
Assessing overall cost-effectiveness of treatment intensification with insulin
A composite endpoint (a combination of multiple single endpoints) can be useful in clinical trials to evaluate treatment of diseases with more than one important outcome. Benefits of therapy (eg, improved glycemic control) can be offset by negative outcomes (eg, treatment-specific adverse events)70 and considering one endpoint in isolation may give an incomplete or biased view of the overall benefit. In people with T2D, optimal treatment should provide effective glycemic control with a low risk of hypoglycemia or weight gain,71 and both these endpoints should be considered when assessing the outcomes and costs of insulin-based therapies.
Randomized, controlled trials of insulin therapies and theoretical therapy models have reported different composite endpoints when assessing cost-effectiveness. Studies of insulin glargine have most commonly used a composite endpoint of the proportion of patients reaching their target without nocturnal hypoglycemia,72 whereas studies of insulin detemir have reported the proportion of patients reaching targets without any episode of hypoglycemia.73
Conclusions
Given the rising incidence of T2D and the burden on healthcare services, HE evaluations of the management of T2D are becoming increasingly relevant worldwide. HE studies in numerous countries have shown that hospital inpatient care (mostly due to diabetes complications) accounts for about half of the total expenditure for T2D, while diabetes medication and supplies account for a much smaller percentage. Thus, diabetes complications are not only detrimental to QoL and long-term prognosis but also account for a disproportionate share of the total cost of managing T2D.
Clinical studies have demonstrated that intensification of treatment to achieve stricter glycemic control and thereby reduce or prevent complications may be one of the most cost-effective interventions for T2D patients with inadequate glycemic control. The studies reviewed here suggest that earlier introduction of insulin therapy may be more cost-effective than prescription of multiple oral therapies with or without incretin therapy. However, adverse events associated with insulin therapy, especially hypoglycemia and weight gain, may offset to some extent the clinical and economic benefit. Although questions remain as to when to initiate insulin and to what extent one insulin analog may be superior to another, in patients with T2D exhibiting poor glycemic control the data reviewed here suggest that treatment with an insulin analog will improve medical outcomes and is cost-effective.
Expert Opinion
As the prevalence of T2D continues to rise, increased pressure on healthcare resources and escalating costs are unavoidable. Decision makers must select interventions that help patients achieve glycemic targets and avoid long-term complications, while also providing value for money. First-line therapy involving metformin along with lifestyle modification (diet, exercise, and weight loss) is typically low cost, but many patients fail to meet glycemic targets on this regimen and require treatment intensification. Although addition of insulin is an option at this stage, many clinicians prefer to recommend a second and even a third OAD if patients still fail to meet targets. However, these patients may be spending a prolonged period in a hyperglycemic state,36,49 increasing both their risk of serious vascular complications and their use of healthcare resources in the long term.
Insulin remains the most effective anti-glycemic therapy in T2D, but the timing of the initiation of insulin treatment is a topic of considerable debate. Earlier use of insulin could reduce and/or delay diabetes complications,52 which would help cut the largest cost in T2D. It has been argued that initiation of insulin is more resource intensive (particularly in terms of clinician time and overcoming patient reluctance) and thus more expensive to initiate than oral therapies. But given that most patients with T2D will ultimately require insulin,53 treatment initiation is not likely to be an avoidable cost. If benefit is to be maximized and cost minimized, insulin treatment must be individualized and self-monitored to avoid hypoglycemia and/or weight gain. Use of insulin glargine or detemir rather than NPH insulin may be useful in this regard; both long-acting analogs are associated with fewer episodes of hypoglycemia9 and insulin detemir with less weight gain.44 More finely tuned guidance concerning the choice of insulin and the ideal timing of initiation require further research.
Five-year View
The global cost of treating T2D is projected to increase over the next five years, reaching approximately €375 billion by 2030.1 Minimizing this cost while improving outcomes will be a major challenge. Unfortunately, many individuals remain unable to make the required long-term changes in their behavior and lifestyle despite investment in educational programs.29 Improvements in outcomes will most likely come from new treatments and better use of existing treatments. In particular, recommendations on the choice of second-line therapy should become clearer in terms of both clinical benefit and cost, and clinical experience with newer agents, such as SGLT2 inhibitors, should provide insight into their place in treatment algorithms.
Earlier insulin initiation may prove more beneficial in the future as new insulin formulations offering better control, fewer adverse events, and easier management of T2D become available. These formulations include ultra-long-acting analogs that have flatter and more consistent metabolic effects and improved adverse event profiles. Insulin degludec, for example, is a novel insulin analog now in clinical use that produces a longer duration of action with varied daily dose timing.74 In addition, degludec is associated with a lower incidence of hypoglycemia than insulin glargine. Longer duration of action and reduced adverse events have also been achieved by conjugating insulin with polyethylene glycol (PEG) in PEGylated insulin lispro,75 which is currently in development. In addition, further innovations blending ultra-long-acting insulin with a short-acting version may offer better postprandial glycemic control.76 The potential advantages these agents have over existing basal insulins suggest that they may have an important role to play in future T2D management.
Key Issues
Diabetes complications are an important cost driver in T2D management; patients with complications incur costs up to 250% higher than patients without complications.
The cost of glucose-lowering drug therapy for T2D is small compared with the cost of managing diabetes complications (18% vs 40–60%, respectively, of the total cost).
Intensive blood glucose control reduces the cost of complications compared to conventional management by more than enough to offset the increase in treatment costs.
Treatment-related adverse events such as hypoglycemia and weight gain can be associated with significant healthcare costs and reduced QoL.
Earlier introduction of insulin therapy may result in more effective blood glucose control, a reduction in the severity and/or delayed onset of diabetes complications, and improved patient QoL.
Hypoglycemia and weight gain associated with insulin therapy may offset to some extent the clinical and economic benefit.
Both insulin glargine and insulin detemir are associated with a lower frequency of hypoglycemia than NPH insulin; insulin detemir is associated with less weight gain.
Treatment with an insulin analog may improve medical outcomes and is cost-effective in patients with T2D with poor glycemic control.
Acknowledgments
Editorial support was provided by Matt Booth, PhD, and Bill Kadish, MD, of PAREXEL MMS, and this support was sponsored by Novo Nordisk A/S, Bagsværd, Denmark.
Footnotes
Estimated currency conversion based on average exchange rate in year of study.
ACADEMIC EDITOR: Nigel Irwin, Editor in Chief
FUNDING: Editorial support was provided by PAREXEL MMS, and sponsored by Novo Nordisk A/S, Bagsværd, Denmark. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: A Liebl has received travel support and various board membership, consultancy, and lectureship fees from AstraZeneca, Bayer, Boehringer Ingelheim, Lilly, Medtronic, MSD, Novo Nordisk, and Roche. K Khunti has acted as a consultant and speaker for Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, and Merck Sharp & Dohme. He has received grants in support of investigator and investigator-initiated trials from Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Pfizer, Boehringer Ingelheim, and Merck Sharp & Dohme. J-F Yale has received honoraria for lectures and advisory boards from Novo Nordisk, Lilly, Sanofi-Aventis, Merck, BMS, AstraZeneca, Boehringer Ingelheim, Novartis, Medtronic, Takeda, and GSK. D Orozco-Beltran has been a member of advisory boards or received honoraria for lectures from Novo Nordisk, Lilly, Sanofi-Aventis, MSD, and Boehringer Ingelheim.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Agreed the manuscript concept and content structure at project initiation: AL, KK, DOB, JFY. Analyzed the data: AL, KK, DOB, JFY. Wrote the first draft of the manuscript: AL, KK, DOB, JFY, with editorial support from PAREXEL MMS. Contributed to the writing of the manuscript: AL, KK, DOB, JFY. Agreed with manuscript results and conclusions: AL, KK, DOB, JFY. Jointly developed the structure and arguments for the paper: AL, KK, DOB, JFY. Made critical revisions and approved final version: AL, KK, DOB, JFY. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2011;26(1):28–35. doi: 10.1093/ndt/gfq576. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Diabetes Factsheet. Geneva: World Health Organization; 2013. [Google Scholar]
- 3.Gillies CL, Lambert PC, Abrams KR, et al. Different strategies for screening and prevention of type 2 diabetes in adults: cost effectiveness analysis. BMJ. 2008;336(7654):1180–1185. doi: 10.1136/bmj.39545.585289.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie CJ, Gale EA, Poole CD. Estimation of primary care treatment costs and treatment efficacy for people with Type 1 and Type 2 diabetes in the United Kingdom from 1997 to 2007*. Diabet Med. 2010;27(8):938–948. doi: 10.1111/j.1464-5491.2010.03040.x. [DOI] [PubMed] [Google Scholar]
- 5.Massi-Benedetti M. The cost of diabetes Type II in Europe: the CODE-2 Study. Diabetologia. 2002;45(7):S1–S4. doi: 10.1007/s00125-002-0860-3. [DOI] [PubMed] [Google Scholar]
- 6.Liebl A, Neiss A, Spannheimer A, et al. Complications, co-morbidity, and blood glucose control in type 2 diabetes mellitus patients in Germany—results from the CODE-2 study. Exp Clin Endocrinol Diabetes. 2002;110(1):10–16. doi: 10.1055/s-2002-19988. [DOI] [PubMed] [Google Scholar]
- 7.Bottomley JM, T2ARDIS Steering Committee Managing care of type 2 diabetes. Learnings from T2ARDIS. Br J Diabetes Vasc Dis. 2001;1:68–72. [Google Scholar]
- 8.Williams R, Van Gaal L, Lucioni C. Assessing the impact of complications on the costs of Type II diabetes. Diabetologia. 2002;45(7):S13–S17. doi: 10.1007/s00125-002-0859-9. [DOI] [PubMed] [Google Scholar]
- 9.Waugh N, Cummins E, Royle P, et al. Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2010;14(36):1–248. doi: 10.3310/hta14360. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez GF, Mavros P, Nocea G, Alemao E, Alexander CM, Yin D. Glycaemic control among patients with type 2 diabetes mellitus in seven European countries: findings from the real-life effectiveness and care patterns of diabetes management (RECAP-DM) study. Diabetes Obes Metab. 2008;10:8–15. doi: 10.1111/j.1463-1326.2008.00881.x. [DOI] [PubMed] [Google Scholar]
- 11.Ramsdell JW, Braunstein SN, Stephens JM, Bell CF, Botteman MF, Devine ST. Economic model of first-line drug strategies to achieve recommended glycaemic control in newly diagnosed type 2 diabetes mellitus. Pharmacoeconomics. 2003;21(11):819–837. doi: 10.2165/00019053-200321110-00005. [DOI] [PubMed] [Google Scholar]
- 12.Grunberger G. The importance of early insulin adoption in type 2 diabetes management. Internet J Fam Pract. 2008;7(2) Available at: https://ispub.com/IJFP/7/2/5172. [Google Scholar]
- 13.Jonsson B. Revealing the cost of type II diabetes in Europe. Diabetologia. 2002;45(7):S5–S12. doi: 10.1007/s00125-002-0858-x. [DOI] [PubMed] [Google Scholar]
- 14.Williams R, Baxter H, Bottomley J, et al. CODE-2* UK: our contribution to a European study of the costs of type 2 diabetes. Practical Diabetes Int. 2001;18:235–238. [Google Scholar]
- 15.Gray A, Fenn P, McGuire A. The cost of insulin-dependent diabetes mellitus (IDDM) in England and Wales. Diabet Med. 1995;12(12):1068–1076. doi: 10.1111/j.1464-5491.1995.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 16.Moore P. Type 2 diabetes is a major drain on resources. BMJ. 2000;320(7237):732. [PMC free article] [PubMed] [Google Scholar]
- 17.This is Money website Historic inflation calculator. 2011. Available at: http://www.thisismoney.co.uk/historic-inflation-calculator.
- 18.Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29(7):855–862. doi: 10.1111/j.1464-5491.2012.03698.x. [DOI] [PubMed] [Google Scholar]
- 19.Liebl A, Spannheimer A, Reitberger U, Gortz A. Costs of long-term complications in type 2 diabetes patients in Germany. Results of the CODE-2 Study. Med Klin (Munich) 2002;97(12):713–719. doi: 10.1007/s00063-002-1215-z. [DOI] [PubMed] [Google Scholar]
- 20.Mata M, Antonanzas F, Tafalla M, Sanz P. The cost of type 2 diabetes in Spain: the CODE-2 study. Gac Sanit. 2002;16(6):511–520. doi: 10.1016/s0213-9111(02)71973-0. [DOI] [PubMed] [Google Scholar]
- 21.Xie X, Vondeling H. Cost-utility analysis of intensive blood glucose control with metformin versus usual care in overweight type 2 diabetes mellitus patients in Beijing, P.R. China. Value Health. 2008;11(suppl 1):S23–S32. doi: 10.1111/j.1524-4733.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- 22.Clarke PM, Gray AM, Briggs A, Stevens RJ, Matthews DR, Holman RR. Cost-utility analyses of intensive blood glucose and tight blood pressure control in type 2 diabetes (UKPDS 72) Diabetologia. 2005;48(5):868–877. doi: 10.1007/s00125-005-1717-3. [DOI] [PubMed] [Google Scholar]
- 23.Gray A, Clarke P, Farmer A, Holman R. Implementing intensive control of blood glucose concentration and blood pressure in type 2 diabetes in England: cost analysis (UKPDS 63) BMJ. 2002;325(7369):860. doi: 10.1136/bmj.325.7369.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray A, Raikou M, McGuire A, et al. Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: economic analysis alongside randomised controlled trial (UKPDS 41). United Kingdom Prospective Diabetes Study Group. BMJ. 2000;320(7246):1373–1378. doi: 10.1136/bmj.320.7246.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics. 2008;26(9):733–744. doi: 10.2165/00019053-200826090-00004. [DOI] [PubMed] [Google Scholar]
- 26.Boren SA, Fitzner KA, Panhalkar PS, Specker JE. Costs and benefits associated with diabetes education. Diabetes Educator. 2009;35(1):72–96. doi: 10.1177/0145721708326774. [DOI] [PubMed] [Google Scholar]
- 27.Gillett M, Dallosso HM, Dixon S, et al. Delivering the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cost effectiveness analysis. BMJ. 2010;341:c4093. doi: 10.1136/bmj.c4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2009;1:CD005268. doi: 10.1002/14651858.CD005268.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N. The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation. Health Technol Assess. 2003;7(22):iii, 1–iii190. doi: 10.3310/hta7220. [DOI] [PubMed] [Google Scholar]
- 30.Davies MJ, Heller S, Skinner TC, et al. Diabetes Education and Self Management for Ongoing and Newly Diagnosed Collaborative. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ. 2008;336(7642):491–495. doi: 10.1136/bmj.39474.922025.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khunti K, Gray LJ, Skinner T, et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow-up of a cluster randomised controlled trial in primary care. BMJ. 2012;344:e2333. doi: 10.1136/bmj.e2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montori VM, Fernandez-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Intern Med. 2009;150(11):803–808. doi: 10.7326/0003-4819-150-11-200906020-00008. [DOI] [PubMed] [Google Scholar]
- 33.Boutati EI, Raptis SA. Self-monitoring of blood glucose as part of the integral care of type 2 diabetes. Diabetes Care. 2009;32(suppl 2):S205–S210. doi: 10.2337/dc09-S312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clar C, Barnard K, Cummins E, Royle P, Waugh N. Self-monitoring of blood glucose in type 2 diabetes: systematic review. Health Technol Assess. 2010;14(12):1–140. doi: 10.3310/hta14120. [DOI] [PubMed] [Google Scholar]
- 35.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Clin Diabetes. 2009;27(1):4–16. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvert MJ, McManus RJ, Freemantle N. Management of type 2 diabetes with multiple oral hypoglycaemic agents or insulin in primary care: retrospective cohort study. Br J Gen Pract. 2007;57(539):455–460. [PMC free article] [PubMed] [Google Scholar]
- 37.Heine RJ, Diamant M, Mbanya JC, Nathan DM. Management of hyperglycaemia in type 2 diabetes: the end of recurrent failure? BMJ. 2006;333(7580):1200–1204. doi: 10.1136/bmj.39022.462546.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Philippe J, Raccah D. Treating type 2 diabetes: how safe are current therapeutic agents? Int J Clin Pract. 2009;63(2):321–332. doi: 10.1111/j.1742-1241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 39.Burke GL, Bertoni AG, Shea S, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168(9):928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Look AHEAD, Research Group, Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prospective Studies Collaboration. Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nauck M, Frid A, Hermansen K, LEAD-2 Study Group et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valentine WJ, Palmer AJ, Lammert M, Langer J, Brandle M. Evaluating the long-term cost-effectiveness of liraglutide versus exenatide BID in patients with type 2 diabetes who fail to improve with oral antidiabetic agents. Clin Ther. 2011;33(11):1698–1712. doi: 10.1016/j.clinthera.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 44.Valentine WJ, Goodall G, Aagren M, Nielsen S, Palmer AJ, Erny-Albrecht K. Evaluating the cost-effectiveness of therapy conversion to insulin detemir in patients with type 2 diabetes in Germany: a modelling study of long-term clinical and cost outcomes. Adv Ther. 2008;25(6):567–584. doi: 10.1007/s12325-008-0069-z. [DOI] [PubMed] [Google Scholar]
- 45.Brandle M, Azoulay M, Greiner RA. Cost-effectiveness and cost-utility of insulin glargine compared with NPH insulin based on a 10-year simulation of long-term complications with the diabetes mellitus model in patients with type 2 diabetes in Switzerland. Int J Clin Pharmacol Ther. 2007;45(4):203–220. doi: 10.5414/cpp45203. [DOI] [PubMed] [Google Scholar]
- 46.Rosenblum MS, Kane MP. Analysis of cost and utilization of health care services before and after initiation of insulin therapy in patients with type 2 diabetes mellitus. J Manag Care Pharm. 2003;9(4):309–316. doi: 10.18553/jmcp.2003.9.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodall G, Sarpong EM, Hayes C, Valentine WJ. The consequences of delaying insulin initiation in UK type 2 diabetes patients failing oral hyperglycaemic agents: a modelling study. BMC Endocr Disord. 2009;9:19. doi: 10.1186/1472-6823-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liebl A, Breitscheidel L, Nicolay C, Happich M. Direct costs and health-related resource utilisation in the 6 months after insulin initiation in German patients with type 2 diabetes mellitus in 2006: INSTIGATE study. Curr Med Res Opin. 2008;24(8):2349–2358. doi: 10.1185/03007990802292728. [DOI] [PubMed] [Google Scholar]
- 49.Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411–3417. doi: 10.2337/dc13-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norton E. The business of intensive insulin therapy for type 2 diabetes patients: where it all began for me. J Diabetes Sci Technol. 2009;3(6):1521–1523. doi: 10.1177/193229680900300635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaefer J. Patient and physician barriers to instituting insulin therapy: a case-based overview. Insulin. 2007;2(suppl 2):S41–S46. [Google Scholar]
- 52.Hisashige A, Katayama T, Mikasa H. Costs and effectiveness of insulin therapy for type 2 diabetes. Value Health. 2001;4(2):113. [Google Scholar]
- 53.Mudaliar S, Edelman SV. Insulin therapy in type 2 diabetes. Endocrinol Metab Clin North Am. 2001;30(4):935–982. doi: 10.1016/s0889-8529(05)70222-x. [DOI] [PubMed] [Google Scholar]
- 54.Asche CV, Shane-McWhorter L, Raparla S. Health economics and compliance of vials/syringes versus pen devices: a review of the evidence. Diabetes Technol Ther. 2010;12(S1):S101–S108. doi: 10.1089/dia.2009.0180. [DOI] [PubMed] [Google Scholar]
- 55.Peyrot M, Rubin RR, Lauritzen T, et al. International DAWN Advisory Panel. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28(11):2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 56.Leichter S. Is the use of insulin analogues cost-effective? Adv Ther. 2008;25(4):285–299. doi: 10.1007/s12325-008-0043-9. [DOI] [PubMed] [Google Scholar]
- 57.Lee LJ, Yu AP, Johnson SJ, et al. Direct costs associated with initiating NPH insulin versus glargine in patients with type 2 diabetes: a retrospective database analysis. Diabetes Res Clin Pract. 2010;87(1):108–116. doi: 10.1016/j.diabres.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 58.National Institute for Health and Clinical Excellence (NICE) Type 2 Diabetes: Newer Agents for Blood Glucose Control in Type 2 Diabetes NICE Website Guidance Pages. London: NICE; 2009. [PubMed] [Google Scholar]
- 59.Jonsson L, Bolinder B, Lundkvist J. Cost of hypoglycemia in patients with type 2 diabetes in Sweden. Value Health. 2006;9(3):193–198. doi: 10.1111/j.1524-4733.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 60.Lundkvist J, Berne C, Bolinder B, Jonsson L. The economic and quality of life impact of hypoglycemia. Eur J Health Econ. 2005;6(3):197–202. doi: 10.1007/s10198-005-0276-3. [DOI] [PubMed] [Google Scholar]
- 61.Holstein A, Plaschke A, Egberts EH. Incidence and costs of severe hypoglycemia. Diabetes Care. 2002;25(11):2109–2110. doi: 10.2337/diacare.25.11.2109. [DOI] [PubMed] [Google Scholar]
- 62.Yu AP, Wu EQ, Birnbaum HG, et al. Short-term economic impact of body weight change among patients with type 2 diabetes treated with antidiabetic agents: analysis using claims, laboratory, and medical record data. Curr Med Res Opin. 2007;23(9):2157–2169. doi: 10.1185/0300799007X219544. [DOI] [PubMed] [Google Scholar]
- 63.Dennett SL, Boye KS, Yurgin NR. The impact of body weight on patient utilities with or without type 2 diabetes: a review of the medical literature. Value Health. 2008;11(3):478–486. doi: 10.1111/j.1524-4733.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- 64.Khunti K, Davies M. Glycaemic goals in patients with type 2 diabetes: current status, challenges and recent advances. Diabetes Obes Metab. 2010;12(6):474–484. doi: 10.1111/j.1463-1326.2009.01186.x. [DOI] [PubMed] [Google Scholar]
- 65.Cavan DA, Ziegler R, Cranston I, et al. Automated bolus advisor control and usability study (ABACUS): does use of an insulin bolus advisor improve glycaemic control in patients failing multiple daily insulin injection (MDI) therapy? [ NCT01460446] BMC Fam Pract. 2012;(13):102. doi: 10.1186/1471-2296-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reach G, Zerrouki A, Leclercq D, d’Ivernois JF. Adjusting insulin doses: from knowledge to decision. Patient Educ Couns. 2005;56(1):98–103. doi: 10.1016/j.pec.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Hammer M, Lammert M, Mejias SM, Kern W, Frier BM. Costs of managing severe hypoglycaemia in three European countries. J Med Econ. 2009;12(4):281–290. doi: 10.3111/13696990903336597. [DOI] [PubMed] [Google Scholar]
- 68.Leese GP, Wang J, Broomhall J, et al. DARTS/MEMO Collaboration. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26(4):1176–1180. doi: 10.2337/diacare.26.4.1176. [DOI] [PubMed] [Google Scholar]
- 69.Brod M, Busk AK, Kragh N, Christensen TE. Underestimated impact of non-severe nocturnal hypoglycemic events (NHEs) on patients’ functioning and well being: approximately 30% of events result in work absenteeism and productivity loss. Diabetes. 2011;60(suppl 1):A329. [Google Scholar]
- 70.McEwan P, Evans M, Kan H, Bergenheim K. Understanding the inter-relationship between improved glycaemic control, hypoglycaemia and weight change within a long-term economic model. Diabetes Obes Metab. 2010;12(5):431–436. doi: 10.1111/j.1463-1326.2009.01184.x. [DOI] [PubMed] [Google Scholar]
- 71.Dailey G, Strange P. Lower severe hypoglycemia risk: insulin glargine versus NPH insulin in type 2 diabetes. Am J Manag Care. 2008;14(1):25–30. [PubMed] [Google Scholar]
- 72.Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 73.Blonde L, Merilainen M, Karwe V, Raskin P. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets—the TITRATE study. Diabetes Obes Metab. 2009;11(6):623–631. doi: 10.1111/j.1463-1326.2009.01060.x. [DOI] [PubMed] [Google Scholar]
- 74.Zinman B, Philis-Tsimikas A, Cariou B, et al. NN1250-3579 (BEGIN Once Long) Trial Investigators. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long) Diabetes Care. 2012;35(12):2464–2471. doi: 10.2337/dc12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bergenstal RM, Rosenstock J, Arakaki RF. A randomized, controlled study of once-daily LY2605541, a novel long-acting basal insulin, versus insulin glargine in basal insulin-treated patients with type 2 diabetes. Diabetes Care. 2012;35(11):2140–2147. doi: 10.2337/dc12-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heise T, Tack CJ, Cuddihy R, et al. A new-generation ultra-long-acting basal insulin with a bolus boost compared with insulin glargine in insulin-naive people with type 2 diabetes: a randomized, controlled trial. Diabetes Care. 2011;34(3):669–674. doi: 10.2337/dc10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]


