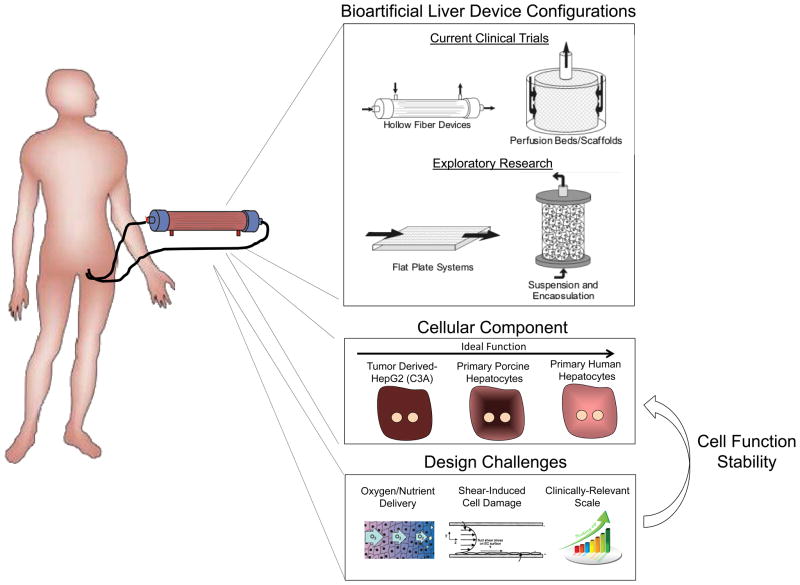
Figure 4. Extracorporeal bioartificial liver devices.
Extracorporeal bioartificial liver devices incorporate functional liver cells and aim to provide an array of important liver functions (detoxification, metabolic, synthetic) for a patient by processing the patient’s blood/plasma outside of the body. This approach could serve as a temporary support and bridge until a liver becomes available for transplantation. Currently, such devices are either in the clinical trial or exploratory research stage. Liver cell-based bioreactor designs primarily fall into four general categories based on device configuration. These include hollow fiber devices, packed beds, flat plate systems, and encapsulation-based reactors. The majority of current clinical trials utilize a hollow fiber design in which cells are positioned outside the fibers and the patient’s blood/plasma is perfused through the fiber lumen. Cell sources include three categories; currently existing tumor cell lines, porcine and human primary hepatocytes, and hepatic cells derived from embryonic stem cells (ESC), induced pluripotent stem cells (iPSC), or reprogrammed from other cell types. Due to their increased availability compared to primary human hepatocytes, primary porcine hepatocytes are the most common cellular component of current bioartificial liver devices. Device design characteristics have been shown to affect the functional stability of the cellular components. Many design challenges exist including the balanced delivery of oxygen and nutrients to the cells, preventing mechanical shear forces from damaging the cells, and clinically relevant scale-up.