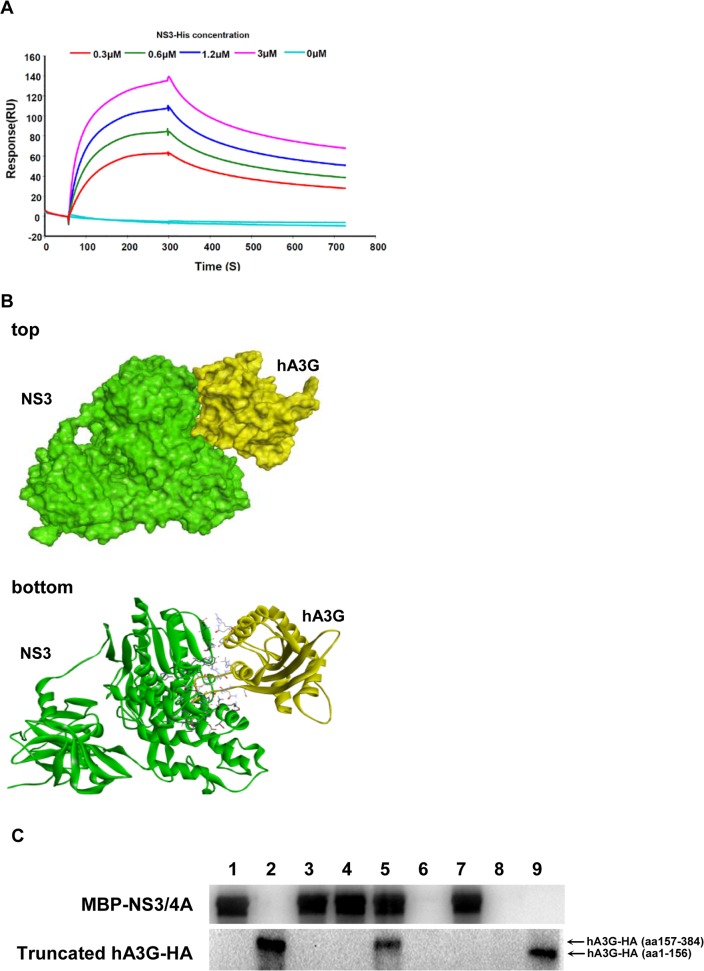
Fig 2. hA3G protein directly bound HCV NS3.
(A) The binding of NS3-His (different concentrations or control) to hA3G-HA were measured with the surface plasmon resonance using BIAcore T100 biosensor system. (B) Computer-simulated docking complex structure between hA3G and NS3 (Pose 11, which was selected from over 2000 poses) were displayed in surface (top) and solid ribbon forms (bottom). (C) C-terminal of hA3G bound MBP-NS3/4A, but N-terminal did not. The MBP-NS3/4A was firstly bound to amylose resin column, followed by interaction with cell lysates of the 293T/17 cells, which were transfected with vectors expressing truncated fraction of hA3G-HA; the western blot analysis for elutes was done with anti-NS3 or anti-HA antibody. Lane 1, MBP-NS3/4A protein control; lane 2, hA3G-HA (aa157-384) positive control; lane 3, negative control (elutes of lysates of 293T/17 cells transfected with control plasmid); lane 4, negative control (elutes of free-cells lysates); lane 5, sample (elutes of lysates of 293T/17 cells transfected with plasmid hA3G-HA (aa157-384)); lane 6, negative control (elutes of lysates of 293T/17 cells transfected with plasmid hA3G-HA (aa157-384), using the amylose resin column with no pre-binding of MBP-NS3/4A); lane 7, sample (elutes of lysates of 293T/17 cells transfected with plasmid hA3G-HA (aa1-156)); lane 8, negative control (lysates of 293T/17cells transfected with plasmid hA3G-HA (aa1-156), using the amylose resin column with no pre-binding of MBP-NS3/4A); lane 9, hA3G-HA (aa1-156) positive control.