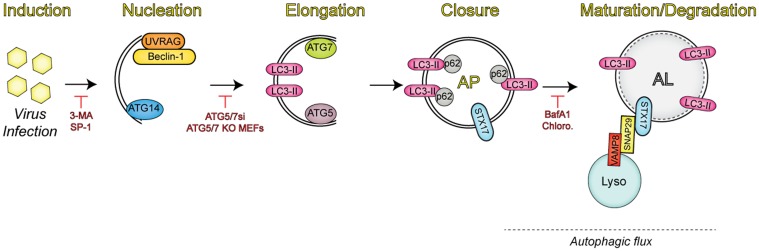
Fig 1. Overview of the autophagic pathway.
Upon infection, viruses trigger the induction of autophagy through a number of mechanisms. Autophagy regulators (i.e., Beclin-1, UVRAG, and ATG14) function in membrane nucleation to form the double-membraned phagophore, which can be blocked via addition of pharmacological inhibitors (3-MA, spautin-1 [SP-1]). Additional autophagy-related proteins (ATG7 and ATG5) mediate the elongation step, in which the phagophore begins to expand until it closes around the material targeted for degradation by sequestration proteins, such as SQSTM/p62. Inhibition of this event is commonly performed through the expression of small interfering RNAs (siRNAs) targeting the autophagic components involved in this process. The completed autophagosome (AP) is then able to fuse with lysosomes (Lyso) via the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex consisting of syntaxin 17 (STX17), SNAP29, and VAMP8. The engulfed contents are then degraded, along with the inner membrane in the newly formed autolysosome (AL), in a process termed autophagic flux. Vesicle acidification inhibitors have been used to block degradation in the AL, given that lysosomal proteases are only active at low pH.