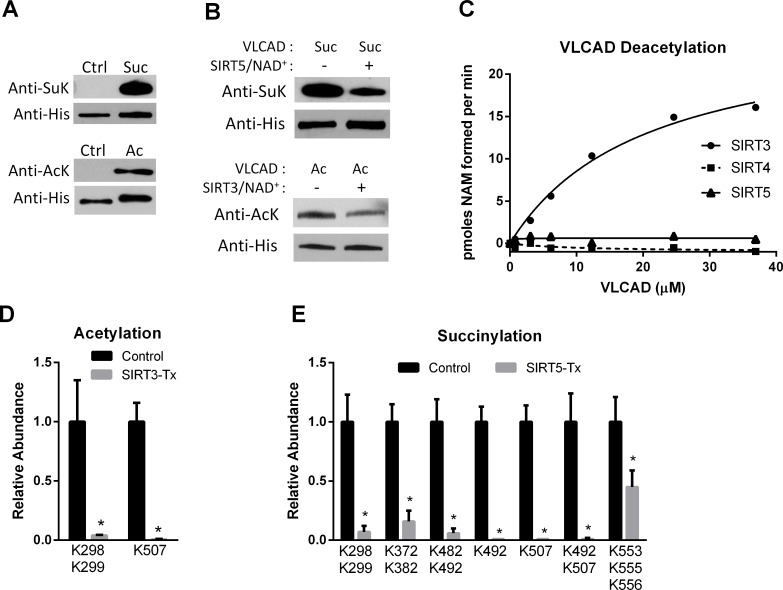
Fig 1. SIRT3 and SIRT5 deacylate VLCAD at overlapping sites.
A) Recombinant, unmodified VLCAD (Ctrl) was subjected to chemical succinylation (top) or acetylation (bottom) which was verified by western blotting with anti-succinyllysine (SuK) or anti-acetyllysine (AcK) antibodies. B) Chemically succinylated (Suc) and acetylated (Ac) VLCAD proteins were reacted with SIRT5 and SIRT3, respectively. Changes in succinylation or acetylation were then evaluated by western blotting, with anti-His blotting as loading control. C) Only SIRT3 reacts with chemically acetylated VLCAD as determined by incubating increasing amounts of acetylated VLCAD with SIRT3, SIRT4, or SIRT5 in the presence of radiolabeled NAD+. Shown are the means of duplicate assays. D) Acetylated VLCAD was treated with SIRT3 or inactive mutant SIRT3 (Control). Quantitative mass spectrometry was used to determine the relative abundance of acetylated peptides. Shown are acetylation sites with >2-fold change. See S1 Dataset for details. E) Succinylated VLCAD was treated with SIRT5 or inactive mutant SIRT5 (Control) and succinylated peptides were quantified by mass spectrometry. Shown are succinylation sites with >2-fold change. See S2 Dataset for details. D and E both depict the means and standard deviations of quadruplicate assays.