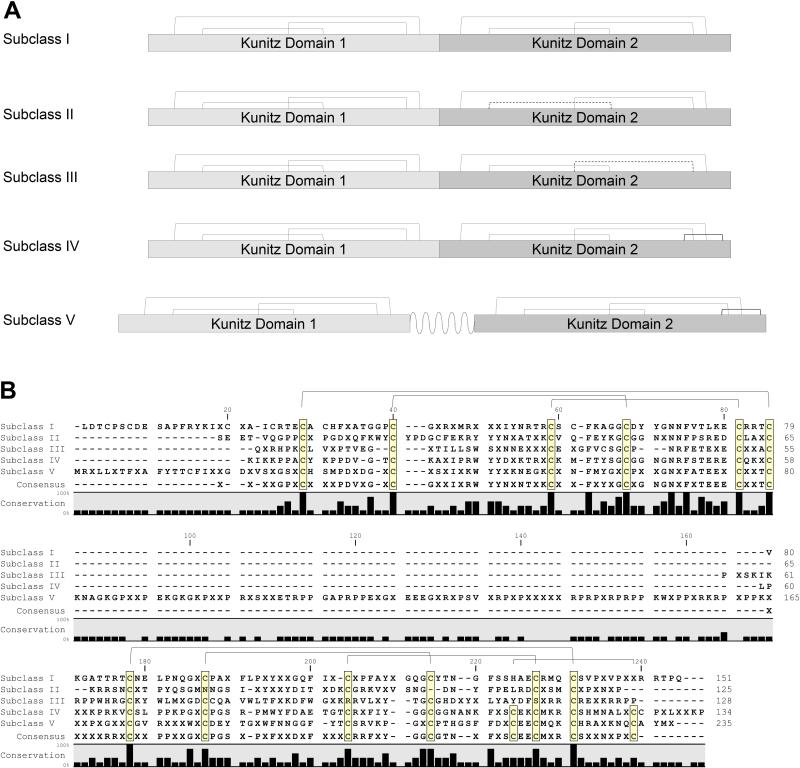
Figure 6. Five subclasses of bilaris proteins.
(A) Schematic representation of the five subclasses of bilaris proteins. Subclass I has two full tandem Kunitz domains, each three conserved disulphide bonds, represented by the solid lines. A typical Kunitz domain disulphide bond pairing is as follows: C1-C6, C2-C4 and C3-C5. Subclass II and III has two missing cysteine residues C2, C4 and C3, C5, respectively, in the second Kunitz domain, resulting in a loss of a disulphide bond. Missing disulphide bonds are represented by the bold dashed line. Subclass IV and V has two extra cysteine residues in the second Kunitz domain, one located between the typical C4 and C5 cysteine residues, and the other after the C6 cysteine residue. This extra disulphide bond is represented by a bold solid line. However, subclass V contains a long inter-domain, proline-rich segment (~ 80 residues and represented by the bold dotted line. (B) Alignment of consensus sequences of the five bilaris subclasses showing conserved disulphide bridges.