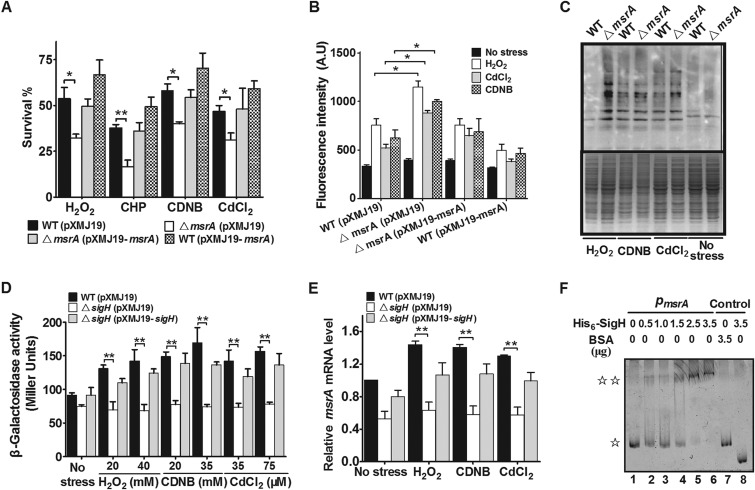
FIG 1.
Requirement for CgMsrA in resistance to multiple oxidative stresses. (A) Survival rates of C. glutamicum strains after being challenged with H2O2 (100 mM), CHP (11 mM), CDNB (70 mM), and CdCl2 (300 μM) for 30 min. (B) The ROS levels in the indicated C. glutamicum strains were measured by using the DCFH-DA fluorescence determination assay after exposure to oxidative reagents. A.U., arbitrary units. (C) Protein carbonyl contents were analyzed by Western blotting with anti-DNP antibody after exposure to various oxidative reagents for 30 min at 30°C. A parallel run stained with Coomassie brilliant blue is shown in the lower gel. Total proteins were extracted from C. glutamicum wild-type cells and msrA mutant cells. (D) β-Galactosidase analyses of msrA promoter activities using the transcriptional PmsrA::lacZ chromosomal fusion reporter expressed in the indicated strains under stress conditions. (E) Quantitative RT-PCR analyses of msrA expression in the indicated strains under stress conditions. (F) EMSA was carried out to analyze interactions between His6-SigH and the msrA promoter. The increasing amounts of His6-SigH used were 0, 0.5, 1.0, 1.5, 2.5, and 3.5 μg. As negative controls, a 240-bp fragment from the msrA coding open reading frame instead of the msrA promoter (lane 8) and BSA instead of His6-SigH (lane 7) were included in the binding assays.  , free DNA;
, free DNA; 
 , major DNA-protein complex. Mean values with standard deviations (SD) (error bars) from at least three repeats are shown. *, P ≤ 0.05; **, P ≤ 0.01.
, major DNA-protein complex. Mean values with standard deviations (SD) (error bars) from at least three repeats are shown. *, P ≤ 0.05; **, P ≤ 0.01.