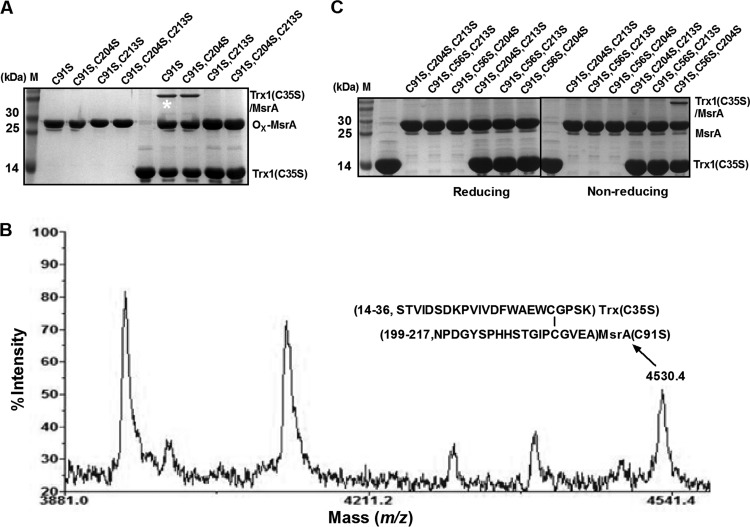
FIG 7.
Interaction between CgMsrA and Trx in vitro. (A) CgMsrA was able to create a stable association with Trx(C35S). Nonreducing SDS-15% PAGE shows each CgMsrA variant incubated with Trx(C35S) in the presence of MetO to generate protein complexes. Ox-MsrA, oxidized MsrA. (B) Analysis of heterodimer by MALDI-TOF MS-MS. A band (labeled with an asterisk) from the nonreducing gel in panel A was excised and treated with trypsin. Only the relevant portion of the mass spectrum is shown. The peaks with m/z 4,530.4 were from dipeptides linked by a disulfide bond between Cys32 in Trx(C35S) and Cys213 in CgMsrA(C91S,C204S). (C) SDS-PAGE analysis of the disulfide-linked protein complexes between CgMsrA variants and Trx(C35S). DTNB-treated Trx(C35S), CgMsrA(C91S,C204S,C213S), CgMsrA(C56S,C91S,C213S), CgMsrA(C56S,C91S,C204S), CgMsrA(C91S,C204S,C213S)/DTNB-treated Trx(C35S), CgMsrA(C56S,C91S,C213S)/DTNB-treated Trx(C35S), and CgMsrA(C56S,C91S,C204S)/DTNB-treated Trx(C35S) were treated or not with DTT. M, protein markers. Addition of DTT led to the disappearance of the disulfide-linked complex.