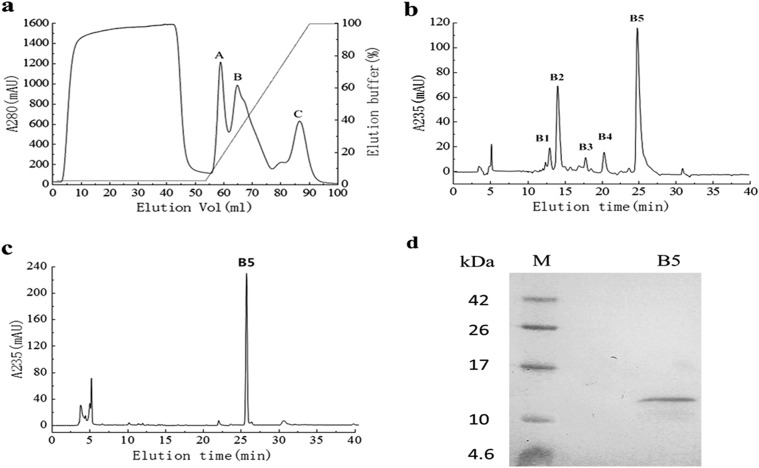
FIG 1.
Purification of PeBL1 from B. laterosporus. (a) The crude protein was loaded on a HiTrap SP FF 5-ml column at a flow rate of 2 ml/min. Three peaks (A, B, and C) were collected, and the target protein was included in peak B. mAU, milli-absorbance units. (b) Chromatography of peak B using a C18 reverse-phase column. The concentration of ACN in the eluted solvent was raised from 20% (vol/vol) to 100% (vol/vol) over 30 min using a linear gradient at a flow rate of 0.2 ml/min. Five main peaks (B1, B2, B3, B4, and B5) were obtained, and peak B5 showed HR activity. (c) We subjected peak B5 to chromatography again under the same conditions, and a single peak was observed. (d) Tricine SDS-PAGE analysis of peak B5 of PeBL1, showing a single band with Coomassie brilliant blue R-250 staining. Lanes: B5, PeBL1; M, protein molecular mass marker.