FIGURE 1:
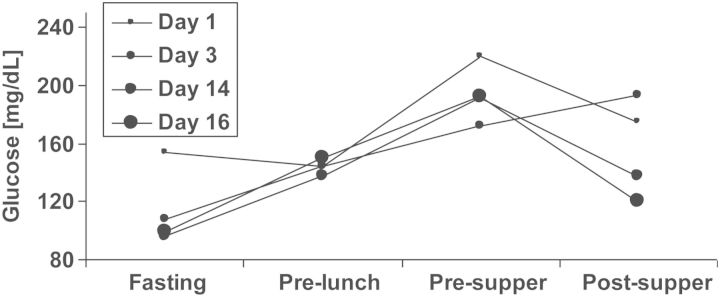
A prototypic blood glucose profile after transplantation. A 67-year-old female with body mass index 27 kg/m2, without diabetes, without family history of diabetes and without hepatitis C infection, had been on haemodialysis for 18 months (lithium-induced nephropathy) before undergoing transplantation with a deceased donor kidney. The patient agreed to participate in the treat-to-target TIP-study [37] and was randomized to the conventional treatment (control) group. HbA1c was 5.0% at baseline. The very early post-transplant glucose profile and the glucose profile in post-transplant Week 3 are displayed. In post-transplant Week 2, the patient received short acting insulin on two consecutive days, but no more insulin corrections were administered and the patient was discharged on post-transplant Day 20. Non-fasting blood glucose ≥200 mg/dL had occurred only during three independent days before discharge. At the first TIP-study control visit on post-transplant Day 87, HbA1c had increased to 7.3%, 2 h glucose value during OGTT was 238 mg/dL, OGTT-derived beta-cell function was poor [insulinogenic index (IGI) = 0.016 nmol insulin/mmol glucose], while insulin sensitivity was not clearly impaired [oral glucose insulin sensitivity (OGIS) index = 300 mL/min/m2]. This patient's decompensation of glucose metabolism had not previously been noticed by measurements of fasting glucose during any of the patient's visits in the outpatient clinic. The patient received an oral antidiabetic agent (sulphonylurea) throughout the end of the study's follow-up as well as 2 years thereafter, when she was additionally contacted. Two consecutive high-dose corticosteroid treatments for acute transplant rejection had been given during post-transplant Weeks 3 and 5, which might have contributed to her rapid impairment of glucose metabolism. As discussed in the text, self-measurements of evening glucose along with early insulin therapy might have uncovered and simultaneously prevented the rapid decline in beta-cell function. Corticosteroid-treated rejections have been shown to associate with NODAT development [45, 53] and may therefore be considered transplant-specific risk factors rather than confounders, as discussed in the text. Fasting: ∼7:30 am, pre-lunch: ∼12:00 am, pre-supper: ∼5:30 pm, post-supper: ∼9:00 pm.
