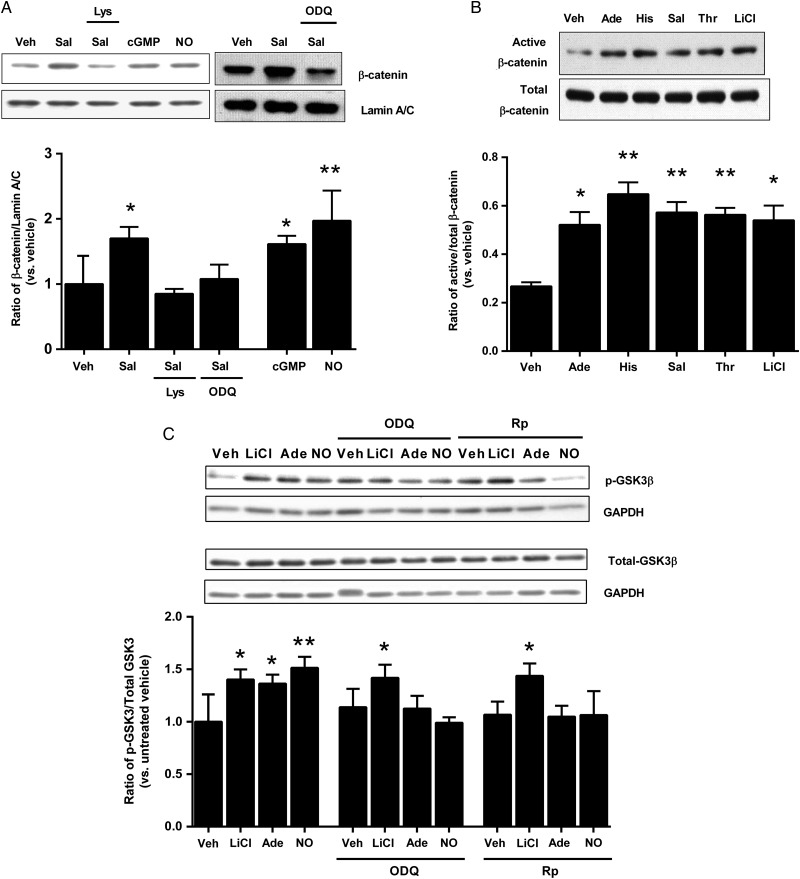
Figure 4.
eNOS activation and NO-cGMP induce nuclear translocation and activation of β-catenin and inhibition of GSK-3β. (A) HUVECs were exposed to salbutamol for 2.5 min, either in the absence or presence of L-lysine (Lys; 1 mmol/L) or ODQ (10 µmol/L), or to 8-bromo-cGMP (cGMP; 10 μmol/L) or spermine NONOate (NO; 10 μmol/L) for 2.5 min. Nuclear extracts were subjected to western blotting for β-catenin and lamin A/C. Results are expressed as the densitometric ratio of β-catenin to lamin A/C, and shown relative to vehicle (*P < 0.05, **P < 0.01 vs. vehicle, one-way ANOVA without repeated measures, n = 5–8 per group). (B) Following exposure to adenosine (Ade; 100 µmol/L), histamine (His; 100 µmol/L), salbutamol (Sal; 1 µmol/L), thrombin (Thr; 1 IU/mL) or LiCl (10 mmol/L) for 2.5 min, whole cell lysates were probed by western blotting for active and total β-catenin. Results are expressed as the ratio of active to total β-catenin (*P < 0.05, **P < 0.01 vs. vehicle, one-way ANOVA with repeated measures; n = 4 per group). (C) HUVECs were exposed to LiCl (10 mmol/L), adenosine (Ade; 100 µmol/L) or spermine NONOate (NO; 10 µmol/L) for 2.5 min, in the presence or absence of ODQ (10 µmol/L) or Rp-8-bromo-guanosine 3′5′-cyclic monophosphorothioate (Rp; 10 µmol/L). Cell lysates were probed for total and phosphorylated GSK-3β. All results are expressed as the densitometric ratio of phosphorylated-GSK-3β/GAPDH to total GSK-3β/GAPDH and shown relative to vehicle (*P < 0.05, **P < 0.01 vs. vehicle, two-way ANOVA; n = 5 per group).