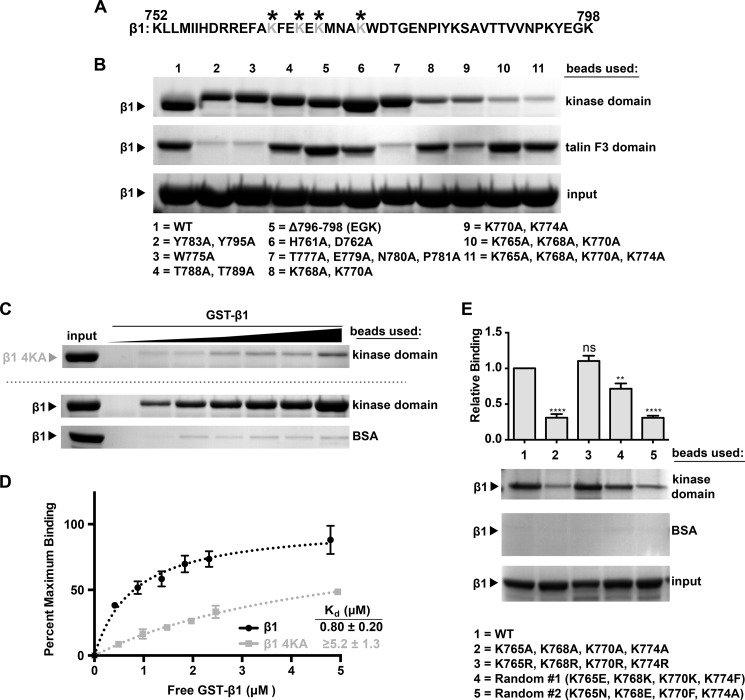
FIGURE 1.
The Arg kinase domain binds to a lysine-rich region in the integrin β1 tail. A, amino acid sequence of the 47-residue integrin β1A cytoplasmic tail. The lysine residues that mediate interactions with Arg are indicated by asterisks. B, binding of Arg kinase domain to various integrin β1A cytoplasmic tail mutants. Agarose beads were coupled to Arg kinase domain (top panel) or the talin F3 domain (middle panel), incubated with GST-β1 tail or GST-β1 tail mutants, and washed, and bound material was analyzed on Bis-Tris PAGE gels and Coomassie Blue-stained. Mutations to the talin F3 domain binding interface (lanes 2, 3, and 7) abrogate talin F3 binding but do not affect Arg kinase domain binding. Similarly, mutations in the known Src nonreceptor tyrosine kinase interface, Δ796–798 (lane 5), and the kindlin-1/kindlin-2 interface, T788A/T789A (lane 4), do not affect Arg kinase binding. However, mutation of four lysine residues in the membrane-proximal half of the tail significantly reduces Arg kinase domain binding (lane 11). C, the concentration dependence of GST-β1 and GST-β1–4KA (K765A/K768A/K770A/K774A) binding to Arg kinase domain was measured. D, one-site specific binding isotherms fit using GraphPad software were set to binding experiments in C. The Kd value for the GST-β1-Arg kinase domain is 0.80 ± 0.20 μm. Saturated binding of Arg kinase domain binding to GST-β1–4KA could not be achieved, and thus we could only place a lower limit of affinity for this interaction of Kd ≥ 5.2 μm. Error bars represent the S.E. from n = 3 for each condition. E, requirements of β1 tail amino acid charge and position for binding the Arg kinase domain. Beads were linked as before with Arg kinase domain or control BSA and incubated in binding assays with WT GST-β1 or GST-β1–4KA, GST-4KR, or two tails in which the 10 amino acids between Lys-765 and Lys-774 were randomized (GST-β1-Random #1 and -Random #2). The 4KR mutant binds at WT levels, whereas both randomized sequences are binding-deficient, indicating that the β1 tail requires positively charged amino acids at positions 765, 768, 770, and 774 for optimal binding to the Arg kinase domain. Error bars represent S.E. from n = 4. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant compared with the WT condition.