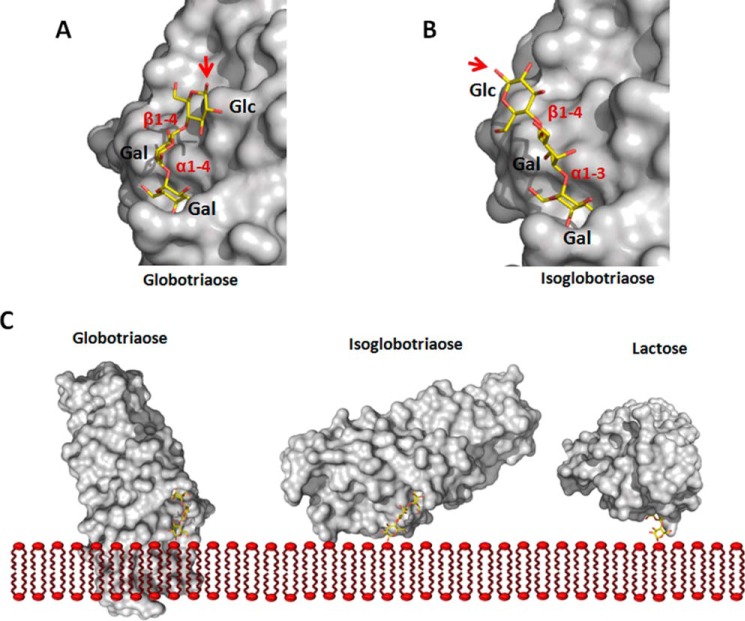
FIGURE 4.
Binding specificity of FaeGad. Globotriaose (A) and isoglobotriaose (B) ligands were docked into the FaeGad carbohydrate binding pocket. Terminal galactose residues substituted with a glycan chain in α1-4 linkage are not functional as FaeGad receptors, whereas those in α1-3 linkage are able to interact with the FaeGad major adhesive subunit (30). The α1-4 linkage of globotriaose positions the glycan chain lateral to the long axis of the FaeGad subunit. A red arrow depicts the C1 atom, which connects the glycan with the sphingolipid part. When the glycosphingolipid is embedded in a membrane, the positioning of the reducing end glycan will result in steric hindrance and abolish the interaction between globotriaose and FaeGad. The α1-3 linkage of isoglobotriaose on the other hand positions the C1 group more at an angle to the subunit's long axis, allowing interaction with membrane-embedded glycosphingolipids without steric hindrance (B and C). A longer glycan chain extends the lipid part further away from the adhesin surface and enhances binding. In the FaeGad-lactose complex, the β1-4 linkage projects the glucose residue perpendicular to the fimbrial shaft and allows the interaction of membrane-embedded lactosylceramide with F4ad fimbriae without steric hindrance (C). In panel C, the orientation of the reducing end glucose monomers of the three glycans is identical. Glycan structures were generated using the GLYCAM web tool. FaeGad is depicted as molecular surface and colored gray. Glycan structures are depicted as stick models with carbon, oxygen and nitrogen atoms colored yellow, red, and blue, respectively.