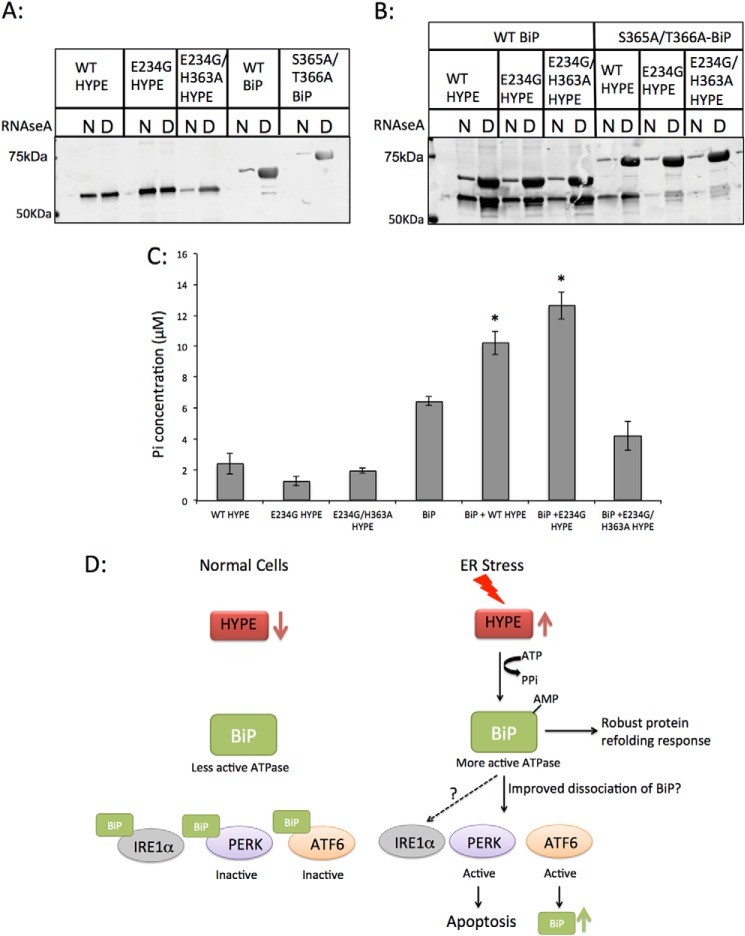
FIGURE 8.
Adenylylation of BiP does not affect BiP's ability to bind misfolded proteins, but it does enhance BiP's ATPase activity. A, purified recombinant WT HYPE or its E234G and E234G/H363A mutants and WT BiP or its S365A/T366A mutant were incubated with native (N) or denatured (D) RNase A bound to Sepharose beads. The proteins were then eluted from beads, separated on SDS-PAGE, and probed for HYPE and BiP. HYPE binds similarly to native and denatured RNase A, although both WT and S365A/T366A BiP bind robustly to denatured RNase. B, unmodified or adenylylated WT and S365A/T366A BiP incubated with native or denatured RNase A show that irrespective of its modification status, BiP robustly binds to denatured RNase A, indicating that BiP's binding to misfolded proteins is not affected by HYPE-mediated adenylylation. C, unmodified or adenylylated WT BiP was assessed for ATPase activity using colorimetric detection of inorganic phosphate released by ATP hydrolysis. BiP modified by WT or E234G HYPE shows significantly higher ATPase activity than unmodified BiP. BiP incubated with E234G/H363A HYPE shows ATPase activity similar to BiP alone, suggesting that HYPE-mediated adenylylation of BiP is responsible for enhancing its ATPase activity. D, model for UPR regulation involving HYPE-mediated adenylylation of BiP. Upon ER stress, HYPE is up-regulated and adenylylates BiP. Adenylylation enhances BiP's ATPase activity, thus enabling it to more efficiently refold misfolded proteins. Adenylylation also enhances BiP's disassociation from PERK and ATF6, thus efficiently activating these pathways. Although our data thus far indicate that IRE1α is not affected by HYPE activity, more work is needed to confirm this *, p < 0.005.