Figure 1.
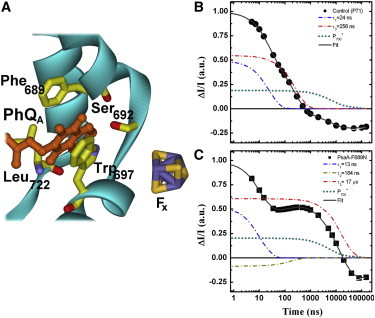
(A) Schematic representation of the binding site for PhQA (orange), highlighting the main cofactor-protein interactions. Also shown is the iron-sulfur center FX (S: yellow, Fe: violet). (B and C) Kinetics of PhQ− oxidation monitored at 395 nm in the control strain (B) or PsaA-F689N mutant (C). Solid symbols are the experimental results and the thick solid line is the fit to a sum of exponential functions. Also shown are the contributions of the decay components ascribed primarily to oxidation of (blue, 24 ns in the WT and 13 ns in the mutant), oxidation of (red, 256 ns in the WT and 17 μs in the mutant), inter-FeS cluster ET (gold, 184 ns in the mutant), and reduction of (the latter is actually the sum of the ∼6 μs and ∼55 μs components). Note that the ∼180 ns component due to inter-FeS cluster ET is not resolvable in the WT. Data are normalized on same total amplitude and same initial intensity. To see this figure in color, go online.
