Figure 7.
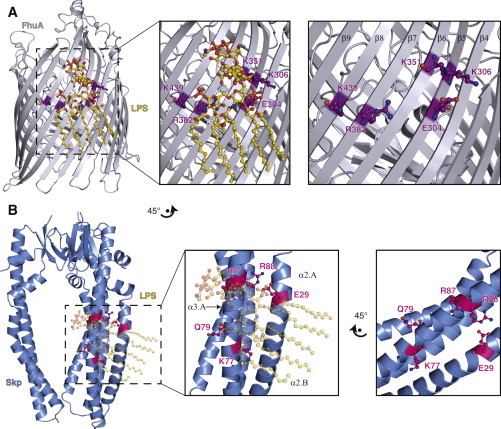
Structural comparison of FhuA-LPS with Skp. (A) Ribbon representation of the FhuA crystal structure (silver) in complex with LPS (gold; PDB 2FCP (36)). Residues E304, K306, K351, R382, and K439, which are part of the FhuA-LPS contact surface, are highlighted in purple. In the right panel, LPS is omitted for clarity to show the structural arrangement of these side chains. (B) Hypothetic structural model of Skp-LPS based on a spatial alignment of the FhuA binding site with conserved Skp residues (23). Ribbon representation of the Skp crystal structure (blue, PDB 1SG2 (24)) with residues E29, K77, Q79, R87, and R88 highlighted in magenta on one Skp-protomer. A zoom is shown in the middle panel. The right panel shows the hypothetic LPS-interaction site rotated by 45°, to be in the same spatial orientation as in FhuA in (A). To see this figure in color, go online.
