Abstract
Hepatitis C virus (HCV)-specific cytotoxic T cell (CTL) response plays a major role in viral control during spontaneous infection resolution. These cells develop an exhausted and pro-apoptotic status during chronic onset, being unable to get rid of HCV. The role of this response in contributing to sustained viral response (SVR) after anti-HCV is controversial. Recent studies show that after successful interferon-based anti-HCV treatment, HCV traces are still detectable and this correlates with a peak of HCV-specific CTL response activation, probably responsible for maintaining SVR by subsequent complete HCV clearing. Moreover, SVR patients’ serum is still able to induce HCV infection in naïve chimpanzees, suggesting that the infection could be under the control of the immune system after a successful treatment, being transmissible in absence of this adaptive response. At least theoretically, treatment-induced viral load decrease could allow an effective HCV-specific CTL response reestablishment. This effect has been recently described with anti-HCV interferon-free regimes, based on direct-acting antivirals. Nevertheless, this is to some extent controversial with interferon-based therapies, due to the detrimental immunoregulatory α-interferon effect on T cells. Moreover, HCV-specific CTL response features during anti-HCV treatment could be a predictive factor of SVR that could have clinical implications in patient management. In this review, the recent knowledge about the role of HCV-specific CTL response in the development of SVR after anti-HCV treatment is discussed.
Keywords: Hepatitis C virus, Chronic hepatitis, Hepatitis C virus-specific cytotoxic T cell response, Treatment, Direct-acting antivirals, Interferon-alpha, Ribavirin, Exhaustion, Apoptosis
Core tip: Hepatitis C virus (HCV)-specific cytotoxic T lymphocyte (CTL) response plays an essential role in controlling acute HCV infection but its implication in treatment-induced viral control is controversial. During interferon/ribavirin treatment, HCV traces persist after sustained viral response (SVR) and this correlates with an activated HCV-specific CTL response, suggesting the necessity of this response to obtain an indefinite viral control. Current data propose that viral suppression during interferon/ribavirin treatment and during direct-acting anti-viral regimes could affect HCV-specific CTL restoration. Moreover, the features of this CTL response during treatment could have a predictive value on SVR outcome.
INTRODUCTION
Hepatitis C virus (HCV)-specific cytotoxic T lymphocytes (CTLs) play an essential role in the natural control of HCV during acute infection[1-4]. These cells are able to recognize HCV infected hepatocytes and destroy them, but they also secrete type I cytokines able to kill the virus in a non-cytopathic manner[5-7] (Figure 1). Nevertheless, in a great percentage of cases the adaptive immune system is unable to get rid of HCV infection, consequently developing in the host a chronic infection[8,9]. Chronic hepatitis C infection is featured by an impaired HCV-specific cytotoxic T cell response unable to control HCV replication[5,10]. This response becomes exhausted in a first step due to the long-lasting high antigenemic burden[11,12], featured by expression of negative co-stimulatory molecules and impaired proliferation and cytokine production[13,14]. Finally, the continuous T cell stimulation could lead to the deletion of these cells by apoptosis induction[15,16]. In these chronic cases, an anti-HCV treatment is compulsory to eliminate the infection, since the adaptive immune system is overwhelmed. Two main kinds of treatments can be currently offered to chronic hepatitis C patients; α-interferon-containing treatments and interferon-free regimes[17]. In both cases, the treatment goal is to maintain an undetectable HCV-RNA by sensitive PCR six months after finishing treatment, which is considered a sustained viral response (SVR). α-Interferon combines an anti-viral effect plus immunomodulatory features[18], while direct-acting antivirals (DAA) have a direct action on HCV replication machinery[19]. In both cases, these treatments could harness the immune system by releasing it from chronic antigenemia. This issue looks clear with DAA but it is more controversial with interferon-containing regimes due to the interferon suppressive effect on T cells[20]. This point will be discussed in this review, along with whether it is necessary to restore an HCV-specific CTL response after anti-HCV treatment to obtain complete viral eradication from hidden sanctuaries in order to maintain an SVR, or on the other hand whether after successful treatment, these cells are not essential anymore because the infection is completed cleared.
Figure 1.
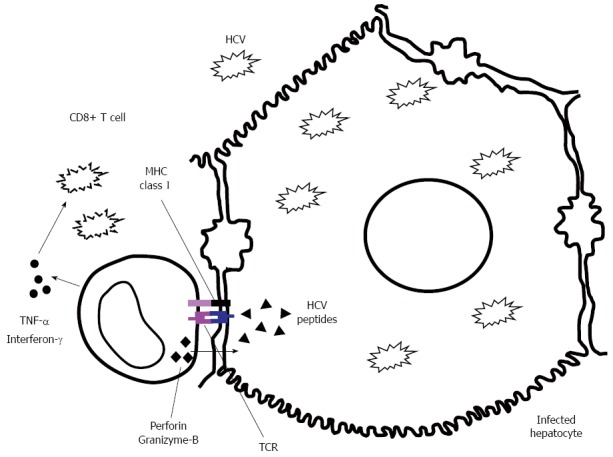
Hepatitis C virus-specific cytotoxic T lymphocyte effector abilities by either direct infected hepatocyte killing through release of perforin and granizyme-B or non-cytopathic hepatitis C virus deletion through γ-interferon and tumor necrosis factors-α secretion. HCV: Hepatitis C virus; TNF: Tumor necrosis factors.
RELATIONSHIP BETWEEN PERISTENT VIRAEMIA AND IMPAIRED SPECIFIC CTL RESPONSE
HCV-specific CTL response is essential to control HCV infection but this becomes dysfunctional (exhausted) and can be deleted during chronic infection due to persistent antigenic stimulus[11,12,15,16]. There are clear demonstrations of the inverse relationship between duration and level of viraemia and T cell responses in murine and human persistent viral infections[21-23]. For instance, in hepatitis B virus infection there is an indirect correlation between viral load and specific-CTL response intensity[24] and interestingly, viral load decrease by nucleos(t)ide analogues is able to restore an HBV-specific CTL response capable of exerting certain effector capacities[25]. In chronic HCV infection, high viraemia leads to exhausted HCV-specific CTLs, featured by impaired proliferation, cytotoxicity and γ-interferon secretion[26]. These cells are characterized by expression of negative co-stimulatory molecules such as PD-1[11,13], down-regulation of the pro-survival IL-7 receptor[15,27] and up-regulation of pro-apoptotic proteins, while this phenotype is not observed in cases with either infection resolution[11,15], or those not targeting the infecting epitope owing to HCV escape mutations[28]. These data suggest that viral load level could modulate the exhausted and pro-apoptotic phenotype on HCV-specific CTLs. Consequently, HCV burden decrease could help in HCV-specific CTL response restoration.
IS IT NECESSARY TO RESTORE THE HCV-SPECIFIC CTL RESPONSE TO OBTAIN A SUSTAINED VIRAL RESPONSE?
Although some studies suggest that HCV is completely eradicated from serum and peripheral blood mononuclear cells of spontaneously recovered or successfully treated patients[29,30], this is a debated topic widely discussed in the literature. Reservoirs of HCV infection could persist after a successful treatment, at least in interferon-containing regimes[31-34]. In fact, negative and positive HCV RNA strands have been demonstrated in liver biopsies from SVR patients, indicating that HCV persisted and replicated in the livers of some sustained responders after treatment with α-interferon plus ribavirin[34]. Other authors have also demonstrated in patients with SVR that small quantities of HCV RNA may persist in liver or macrophages and lymphocytes for up to 9 years after therapy. This continuous viral presence could result in persistence of humoral and cellular immunity for many years after therapy and could present a potential risk for infection reactivation[32]. Moreover, in non-viraemic HCV antibody-positive patients, liver biopsies that are usually abnormal have been shown. Fibrosis was present in most cases with similar inflammatory infiltrate to viraemic cases. The presence of a CD8+ rich inflammatory infiltrate in those cases could suggest an ongoing immune response in the liver, supporting the view that HCV may persist in the liver in some of considered HCV-RNA-negative cases, analyzed with standard PCR techniques[35]. Thus, all these data suggest that at least some SVR patients would not experience a complete HCV infection clearance, despite apparent clinical disease resolution, and in these cases HCV-specific CTL restoration could play an essential role in avoiding infection relapse. In fact, a longitudinal analysis of HCV RNA and HCV-specific CTL response in SVR patients after treatment disclosed that HCV traces persisted several years after end of treatment and interestingly, these HCV RNA peaks matched with a detectable HCV-specific CTL response, expressing activation markers and, suggesting that these cells could keep the virus under control after SVR[36]. Additionally, the sera of these SVR patients could also be infective, since chimpanzees have been infected, using the serum from resolver cases with detectable HCV traces after complete clinical infection resolution due to anti-HCV treatment[37]. It is true that very few cases of HCV reactivation after infection resolution have been described[38,39], in contrast to what happens in HBV infection, mainly in immunosuppressed patients[40]. In fact, during clinical practice, most of the HCV patients who show sustained undetectable viraemia with usual PCR assay develop a permanent clinical control, while in HBV infection is more common the viral reactivation under immune-suppressive conditions. Nevertheless, this does not mandatory mean that after SVR, HCV is completely cleared by interferon/ribavirin treatment. In fact, the data previously summarized show that HCV replication a long period after SVR development can be demonstrated but probably, these viral traces are finally completely deleted by the HCV-specific-CTL response, avoiding thus far the possibility of a late HCV infection reactivation. Thus, checking the quality of the HCV-specific CTL-response would be useful for tracking those patients who develop immunosuppression soon after developing SVR and consequently, could be at risk of recurrence until their immune system is restored[38,39]. On the other hand, in interferon-free regimes, based on DAA combination, there is not yet any data about occult infection after SVR. These therapeutic combinations are highly effective in controlling the infection but what has not been addressed yet is whether adaptive immune response collaboration is necessary to obtain the desired goal. Before treatment, there are naturally occurring resistant variants to DAA combinations[41-43], because of the high mutation rate of HCV during replication, due to the lack of proofreading function in the HCV RNA polymerase[44]. It is true that viral resistance to DAA regimens will occur in a reduced number of virions, but at least statistically, this situation could happen and, in this setting it could be necessary to combine the effect of these powerful drugs with the immune system action to get rid of the minor resistant variants to DAA regimes.
INTERFERON-BASED TREATMENTS
α-Interferon can have a direct antiviral activity by induction of interferon stimulated genes, such as protein kinase R (PKR)[45], oligoadenylate synthetase (OAS)[46], myxovirus resistance protein (Mx)[47], apolipoprotein B mRNAediting enzyme-catalytic polypeptide-like (APOBEC)[48,49] or tripartite motifs (TRIM), which directly inhibit viral replication[18]. Moreover, α-interferon develops an indirect anti-viral effect by affecting the innate and adaptive immune response through expansion of activated specific CD8+ T cells[50-52] and NK cells[52,53], up-regulation of proteins of the antigen presentation machinery[54], maturation of dendritic cells (DC)[55], and augmentation of B-cell responses[51,56]. With respect to adaptive cytotoxic response, α-interferon could boost specific-CTL response by expansion of activated cells[50,57] but also by blocking exhausted status by antigen burden decrease[58,59]. Nevertheless, α-interferon can also have a negative effect on T cell numbers due to regulation of T cell recirculation[20]. The dynamics of HCV viral load during PEG-α-interferon/ribavirin treatment has two phases. First decay is rapid and it is assumed to be owing to PEG-α-interferon direct effect, while second phase decay is slower and it is probably caused by either adaptive immune response[60] or NK cell activation[61,62]. However, NK cells very soon after starting treatment are already activated and, therefore they could act during the first phase decay. Actually, according to mathematical methods, the second decay of HCV viral kinetics during treatment is due to the effect of cellular immune response, mainly specific-CTLs and NK cells[63-65]. NK cells constitute an early host defense against viral pathogens[66,67], eliminating virus-infected cells both directly through cytolytic mechanisms and indirectly by secreting cytokines such as γ-IFN[68]. Although NK cells have been classically viewed as innate immune cells, their effects could extend into periods of adaptive immunity, and hepatic NK cells also demonstrate adaptive immunity to structurally diverse antigens[69,70]. NK cell frequency increases as early as hours following the initiation of antiviral therapy and this is associated with early viral response[61,62], which could contribute to HCV-specific CTL restoration by decreasing initially the viral burden. Interestingly, absence of a strong HCV viral load decrease at week 12 of PEG-α-interferon/ribavirin treatment has 100% negative predictive value of SVR and it is used as a treatment stopping rule[71]. This effect could be related to the impairment of HCV-specific immune response at that point. However, the role of PEG-α-interferon/ribavirin treatment on HCV-specific T cell kinetics has not been completely addressed in-vivo and, it is a controversial issue. In the early phase of infection, α-interferon-based treatment can rescue a polyfunctional long lived HCV-specific CTL response, while this issue is not clear in long-lasting infection[72,73]. A previous paper correlated the development of SVR after PEG-α-interferon/ribavirin treatment with induction of a HCV-multispecific CD4+ Th-1 response[74]. Another study suggested that antiviral therapy-induced viral clearance may be associated with the induction, expansion, and/or recirculation of HCV antigen-specific cytolytic T cells, and it may play a role in the maintenance of a non-viraemic state[75]. Moreover, a longitudinal improvement in NS3-specific CTL response during interferon-based treatment has also been described[76]. Furthermore, a core-specific and NS3-specific CTL response restoration in SVR patients featured by higher frequency and cytotoxicity after α-interferon-based treatment has also been reported[77]. In that work, a peak of specific-CTL response in SVR patients between treatment week 4 and week 12 was observed. A similar finding was stated by Tatsumi et al[78], describing an NS3-specific and core-specific CTL response increase in SVR patients after four weeks of treatment with α-interferon/ribavirin treatment. Nevertheless, another work focused on CD8 and CD4 specific responses did not show a clear association between T cell reactivity and treatment outcome although they found an enhancement of proliferative T-cell responses during therapy[79]. Additionally, a recent retrospective analysis of peripheral blood lymphocyte samples from DITTO study[80] likewise did not show a correlation between restoration of HCV-specific CTL response and SVR after PEG-α-interferon/ribavirin treatment although patients presenting a better HCV-specific CD8 cell proliferative potential at baseline were more likely to present a rapid and sustained viral response[81]. Curiously, a work carried out by our group observed a similar treatment outcome independently of the frequency of peripheral cells at base line. Nevertheless, an improvement of peripheral HCV-specific CTL frequency after 12 wk of treatment correlated with SVR development[60] (Figure 2), in concordance with Tatsumi et al[78] and Caetano et al[77] data. Therefore, treatment could be able to restore HCV-specific CTL numbers, at least in one subpopulation of patients. This finding was not described in Pilli’s work[81] but was suggested in Barnes’s study[79] and it could be due to the type of samples analyzed. In our research[60], directly ex-vivo cells were checked, while in Pilli’s paper[81] frozen cells were retrospectively tested and the thawing process could have affected the more exhausted cells, preventing their detection thus far. On the other hand, investigations analyzing HCV-specific T cell response by γ-interferon-ELISPOT do not support that treatment-induced viral clearance is associated with an enhanced antiviral T cell response[82,83]. The apparent discrepancy between these observations could be explained by the manner in which specific T cells are quantified, since in former studies, cells were recognized by their ability to produce type-I cytokines and, in Larrubia’s work, those cells were visualized directly by HLA-I/epitope multimeric complex labelling. Consequently, the HCV-specific CTL number restoration observed in a subpopulation of infected cases in Larrubia’s study would not be able to secrete γ-interferon yet[15], as a result those cells would not be detected by Barnes et al[79]. Thus, the functionality of these cells should be further analyzed since they could be present but dysfunctional, although a specific proliferative potential improvement during treatment was observed, suggesting a certain degree of functional restoration. Besides, a decrease of programmed cell death protein (PD-1) expression on HCV-specific CTLs after treatment in SVR patients was also described (Figure 3). PD-1 is an immunoreceptor tyrosine-based inhibition motif-containing (ITIM-containing) expressed on activated T cells that mediates hyporesponsiveness[84]. PD-1 expression on HCV-specific CTLs inversely correlates with early and sustained virologic response to IFN-based antiviral therapy[85] and, it is associated with impaired control of in-vitro replication[86]. Therefore, a certain degree of HCV-specific CTL function restoration after treatment in our work was also observed, since the negative co-stimulatory molecule PD-1 was down-regulated in treatment responders[60]. In this work, only patients with a high viral load decrease during the first 12 wk of treatment had detectable HCV-specific CTL at week 12 and this was maintained throughout the treatment (Figure 3). A similar correlation between viral load and T cell response kinetics in other chronic hepatotropic non-cytopathic viral infections has already been shown[24]. Taken together, these data suggest that in chronic hepatitis C cases with conserved HCV-specific T cells, a rapid viral load decrease could suppress the exhausted status on those cells, allowing their peripheral detection. Nevertheless, in patients with either deleted HCV-specific CTLs or with low HCV viral load decrease due to interferon insensitivity it would be impossible to restore a detectable HCV-specific CTL response. As a result, it could be possible to speculate that although type-I interferon associates with lymphopenia induction that could impair specific-T cell number by affecting lymphocyte re-circulation[20], it also allows for an increase in frequency of those cells by favoring clonal expansion and memory formation[87] and by decreasing T cell exhaustion through viral load reduction[24]. The balance between those α-interferon effects would be responsible for the peripheral detection of these cells during treatment, although these cells will still show an exhausted status that will disappear after developing an SVR. Additionally, during interferon based treatment, NK cells have a major role in controlling infection[61,62] and this will help in HCV-specific CTL response restoration after treatment[60,75-78], which will finally contribute to indefinite HCV control and eradication[36]. Consequently, the current information published suggests that in cases with SVR an HCV-specific CTL response improvement during treatment can be observed, although it is still dysfunctional but acquires effector capacities after end of treatment and is probably responsible for destroying viral traces (Figure 3). Unfortunately, there are not data yet about HCV-specific CTL response during treatments combining α-interferon with the new DAA[88], but we already have some information about DAA combination regimes and this will be discussed in the following lines.
Figure 2.
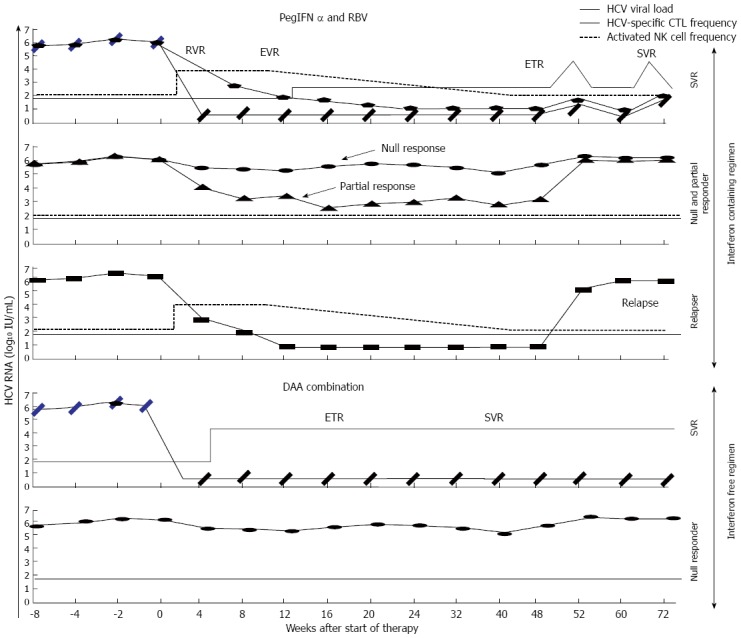
Theoretical hepatitis C virus viral load, hepatitis C virus-specific cytotoxic T lymphocyte frequency and activated natural Killer cell frequency kinetics during anti-hepatitis C virus treatment with interferon-free and interferon-containing regimes, according to the different types of response. PegIFN: Pegylated-a2-interferon; RBV: Ribavirin; DAA: Direct acting antiviral; NK: Natural Killer cell; CTL: Cytotoxic T lymphocyte; HCV: Hepatitis C virus; RVR: Rapid viral response; EVR: Early viral response; ETR: End of treatment response; SVR: Sustained viral response.
Figure 3.
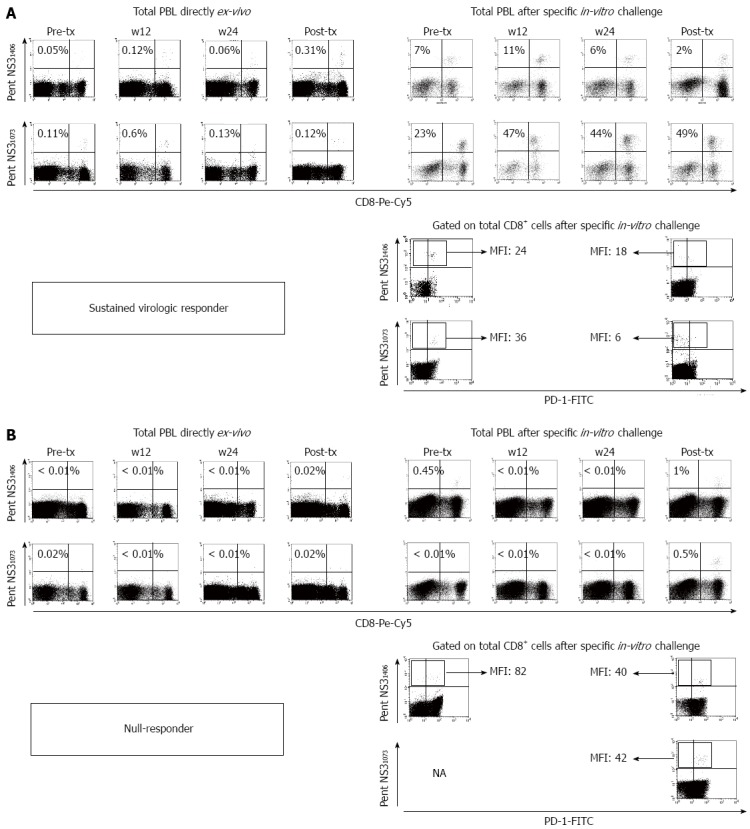
Representative FACS® dot-plots of peripheral blood mononuclear cells from a sustained viral responder (A) and a null responder (B) after treatment with pegylated-α2-interferon plus ribavirin. The dot-plots show the frequency of hepatitis C virus (HCV)-specific cytotoxic T lymphocyte (CTLs) directly ex-vivo and after specific in-vitro challenge, besides the PD-1 phenotype pre- and after treatment. PBL: Peripheral blood lymphocytes; MFI: Mean fluorescence intensity; tx: Treatment; NA: Not available; PentNS31406: Multimeric complexes HLA-A2/NS31406 phycoerithryn labelled; PentNS31073: Multimeric complexes HLA-A2/NS31073 phycoerithryn labelled.
INTERFERON FREE REGIMES
Direct-acting antivirals are able to eliminate infection through affecting the function of different HCV proteins involved in HCV replication, such as NS3/NS4 protease, NS5A complex and NS5B polymerase[17]. Interestingly, DAA combinations could get rid of HCV infection by preventing the effect of naturally occurring resistant variants against one or another single DAA[41-43]. However, it is still not clear whether this treatment is sufficient to eliminate HCV infection or if it is also necessary to restore an efficient HCV-specific CTL response to eradicate the viral traces from specific sanctuaries and to eliminate potential resistant variants to DAA combinations. Recent work has analyzed the role of HCV-specific CTL response in chronic HCV patients treated with interferon-free therapy[89]. In this paper is nicely shown that responder patients to DAA therapy recover a HCV-specific CTL response with the ability to proliferate after antigen encounter, while this is not observed in non-responder cases (Figure 2). These findings are similar to the ones reported in some studies with IFN-based regimens[60,74-78], but more intense probably because in both cases a positive effect of viral load decrease on HCV-specific T cell is present but, in DAA regimens the predominantly negative IFN immune suppressive influence on T cells is avoided[90]. Moreover, this work not only shows an increase in the number of T cells but it also describes an improvement in the quality of the response by down-regulation of negative co-stimulatory molecules and by restoring its cytolytic activity. Thus, this finding supports a role for DAA viral clearance in driving recovery of HCV-specific CTLs but we do not still know if this restoration play a role in controlling HCV infection during treatment or it is only an epiphenomenon. The same consistent adaptive T cell response restoration has been described in chronic HBV infection during nucleos(t)ide analogue treatment, reinforcing these data the idea that, at least in cases without specific-T cell deletion, intense viral load decrease can restore a previously exhausted T cell response[25,91]. According to this observation, it is possible to speculate that specific-CTL restoration during DAA regimes could contribute to the high-sustained cure rates and the low relapse frequency described with this kind of therapies. In Table 1, the studies in favor or against HCV-specific CTL role in SVR after interferon-free and -containing regimes are summarized.
Table 1.
Summary of published works about hepatitis C virus-specific cytotoxic T lymphocyte during anti-hepatitis C virus treatment pointing-out the data in favor and against hepatitis C virus-specific cytotoxic T lymphocyte response restoration either during or after treatment
| Ref. | Year | Treatment | Samples | CTL visualization | In favor | Against |
| Martin et al[89] | 2014 | DAA | Frozen PBMC | Tetramer staining | Restoration of HCV-specific CTL response during treatment in SVR cases | |
| Larrubia et al[60] | 2013 | PEG-IFN/RBV | Fresh PBMC | Pentamer staining | HCV-CTLs detection at week 12 of treatment correlates with infection resolution Detection of PD-1low HCV-specific CTLs after treatment correlates with SVR | |
| Humphreys et al[82] | 2012 | PEG-IFN/RBV | Frozen PBMC | γ-IFN ELISPOT assay | Treatment induced genotype-3a viral clearance is not associated with an enhancement of antiviral T cell response | |
| Tatsumi et al[78] | 2011 | PEG-IFN/RBV | Fresh PBMC | γ-IFN ELISPOT assay | HCV-specific CTL frequency increase between base-line and treatment week 4 was observed in SVR but not in non SVR patients | |
| Barnes et al[83] | 2009 | PEG-IFN/RBV | Frozen PBMC | γ-IFN and IL-2 ELISPOT assay | Not enhanced specific T cell response during high dose IFNa therapy was reported | |
| Caetano et al[77] | 2008 | PEG-IFN/RBV | Fresh PBMC | Pentamer staining | Chronically infected patients who responded to treatment showed a higher HCV-specific CTL frequency than non-responders during and post-treatment Terminally differentiated effector cells increased more rapidly in responders, and their frequency was always higher than in non-responder patients | |
| Golden-Mason et al[85] | 2008 | PEG-IFN/RBV | PBMC | Pentamer staining | Patients with sustained viral response showed a decrease in PD-1 expression on HCV-specific CTLs after therapy completion | |
| Badr et al[73] | 2008 | PEG-IFN | Frozen PBMC | Tetramer staining | Early therapeutic intervention during the acute phase of HCV infection reconstituted a long-lived polyfunctional memory T cell response | |
| Pilli et al[81] | 2007 | PEG-IFN/RBV | Frozen PBMC | γIFN ELISPOT assay and tetramer staining | No significant correlation between HCV-specific CTL kinetics and viral decay during treatment | |
| Morishima et al[75] | 2003 | IFN/RBV | Fresh PBMC | Chromium-51 release assay | Subjects who had completed the treatment and had undetectable HCV RNA levels after therapy had a detectable HCV-specific CTL response more frequently than those who were viraemic | |
| Vertuani et al[76] | 2002 | IFN | Fresh PBMC | Chromium-51 release assay | Increase in the intensity of HCV epitope recognition by HCV-specific CTL after 1 to 5 m of treatment | |
| Barnes et al[79] | 2002 | IFN/RBV | Frozen PBMC | γIFN ELISPOT assay | T cell responses induced during high dose of IFNa treatment appear not to influence the virological outcome |
DAA: Direct acting anti-viral; IFN: Interferon-α2; RBV: Ribavirin; PBMC: Peripheral blood mononuclear cells; γ-IFN: Gamma-interferon; SVR: Sustained viral response.
RESTORATION OF A HCV-SPECIFIC CTL RESPONSE AS PREDECTIVE FACTOR OF SVR
According to the previous data, the extent of T cell reconstitution might be able to be used as a guide to personalize anti-HCV therapy and as a predictive factor of response. A lack of induction of T cells could signal the need for therapy intensification and conversely, a good T cell recovery could allow shorter treatment duration in some patients, with the expectation that any residual virus would be cleared by T cells. These issues have not been completely addressed yet, but there are a few data that will be discussed in the next lines. To address whether restoration of peripheral HCV-specific CTL number could correlate with SVR rate and behave as a treatment response prognosis factor, a multivariate analysis was carried out by our group during interferon-based treatment[60]. In that analysis, the increase of detectable HCV-specific CTLs after 12 wk of treatment was an independent factor related with SVR. Moreover, the predictive value of this variable on early or delayed viral responder (EDVR)[71] genotype-1 cases was also analyzed to understand its role in a hypothetical guided-therapy decision process. Interestingly, all the EDVR genotype-1 samples with detectable HCV-specific CTLs at w12 developed SVR, which means a 100% positive predictive value (PPV). This data could be useful to encourage these patients to finish treatment, but it could also be a decision rule to maintain double therapy in cases with positive cells or to add a DAA in patients without detectable HCV-specific CTLs at w12, which could have interest from an economical point of view in countries restricting the free use of DAA[92,93]. Consequently, the excellent PPV treatment response of HCV-specific CTL detection at w12 could be complementary to the high negative predictive value of a viral load decrease lower than 2log at w12 of PEG-α-interferon/ribavirin treatment, as a strategic tool in the clinical decision process during treatment. We should await similar studies on DAA-based regimes to verify whether HCV-specific CTL restoration during treatment could be used as a response predictive factor and as a tool to guide therapy.
CONCLUSION
Treatment-induced viral load decrease can restore a reactive HCV-specific CTL response during treatment in DAA-based treatments and this can influence the high rate of sustained response observed with this regime. In IFN-based treatment, HCV-specific CTL restoration in some cases can be observed and this issue has an excellent positive predictive value in developing an SVR outcome. In this setting, NK cells develop a major role in viral load decrease that could impact on HCV-specific CTL restoration, although still displaying exhausted features. However, this response reaches a non-exhausted phenotype after treatment in SVR cases and it correlates thereafter with activation peaks coinciding with HCV detection after HCV clinical resolution, suggesting an important role in maintaining SVR and in the complete HCV eradication after treatment. Therefore, taking into account all these data, we can suggest that a potent anti-viral treatment could not be enough alone to obtain a high cure rate, but probably it is also necessary to restore a powerful HCV-CTL response to get that goal.
Footnotes
Supported by “Instituto de Salud Carlos III”, Spain and “European Regional Development Fund, a way of making Europe”, E.U. (PI12/00130) and, Moreno-Cubero E was funded by a research award from “Asociación de Hepatología Translacional” (AHT Research Award 2014), Spain.
Conflict-of-interest: The authors declare that there are no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 19, 2014
First decision: December 4, 2014
Article in press: February 5, 2015
P- Reviewer: Fanning LJ, Rodriguez-Frias F, Takaki A S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
References
- 1.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, Houghton M, Parham P, Walker CM. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- 3.Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, Rosen HR. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol. 2008;82:1827–1837. doi: 10.1128/JVI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyk-Pearson S, Tester IA, Lezotte D, Sasaki AW, Lewinsohn DM, Rosen HR. Differential antigenic hierarchy associated with spontaneous recovery from hepatitis C virus infection: implications for vaccine design. J Infect Dis. 2006;194:454–463. doi: 10.1086/505714. [DOI] [PubMed] [Google Scholar]
- 5.Larrubia JR, Moreno-Cubero E, Lokhande MU, García-Garzón S, Lázaro A, Miquel J, Perna C, Sanz-de-Villalobos E. Adaptive immune response during hepatitis C virus infection. World J Gastroenterol. 2014;20:3418–3430. doi: 10.3748/wjg.v20.i13.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jo J, Aichele U, Kersting N, Klein R, Aichele P, Bisse E, Sewell AK, Blum HE, Bartenschlager R, Lohmann V, et al. Analysis of CD8+ T-cell-mediated inhibition of hepatitis C virus replication using a novel immunological model. Gastroenterology. 2009;136:1391–1401. doi: 10.1053/j.gastro.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Rosen HR. Emerging concepts in immunity to hepatitis C virus infection. J Clin Invest. 2013;123:4121–4130. doi: 10.1172/JCI67714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859–868. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajarizadeh B, Grebely J, Dore GJ. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol. 2013;10:553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 10.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 11.Larrubia JR, Benito-Martínez S, Miquel J, Calvino M, Sanz-de-Villalobos E, González-Praetorius A, Albertos S, García-Garzón S, Lokhande M, Parra-Cid T. Bim-mediated apoptosis and PD-1/PD-L1 pathway impair reactivity of PD1(+)/CD127(-) HCV-specific CD8(+) cells targeting the virus in chronic hepatitis C virus infection. Cell Immunol. 2011;269:104–114. doi: 10.1016/j.cellimm.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 13.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum HE, Pircher H, Thimme R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood NA, Linn ML, Bowen DG. Exhausted or just sleeping: awakening virus-specific responses in chronic hepatitis C virus infection. Hepatology. 2011;54:1879–1882. doi: 10.1002/hep.24602. [DOI] [PubMed] [Google Scholar]
- 15.Larrubia JR, Lokhande MU, García-Garzón S, Miquel J, González-Praetorius A, Parra-Cid T, Sanz-de-Villalobos E. Persistent hepatitis C virus (HCV) infection impairs HCV-specific cytotoxic T cell reactivity through Mcl-1/Bim imbalance due to CD127 down-regulation. J Viral Hepat. 2013;20:85–94. doi: 10.1111/j.1365-2893.2012.01618.x. [DOI] [PubMed] [Google Scholar]
- 16.Radziewicz H, Ibegbu CC, Hon H, Osborn MK, Obideen K, Wehbi M, Freeman GJ, Lennox JL, Workowski KA, Hanson HL, et al. Impaired hepatitis C virus (HCV)-specific effector CD8+ T cells undergo massive apoptosis in the peripheral blood during acute HCV infection and in the liver during the chronic phase of infection. J Virol. 2008;82:9808–9822. doi: 10.1128/JVI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2014. J Hepatol. 2014;61:373–395. doi: 10.1016/j.jhep.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Gibbert K, Schlaak JF, Yang D, Dittmer U. IFN-α subtypes: distinct biological activities in anti-viral therapy. Br J Pharmacol. 2013;168:1048–1058. doi: 10.1111/bph.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Au JS, Pockros PJ. Novel therapeutic approaches for hepatitis C. Clin Pharmacol Ther. 2014;95:78–88. doi: 10.1038/clpt.2013.206. [DOI] [PubMed] [Google Scholar]
- 20.Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108:3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- 21.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 23.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 24.Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL, Williams R, Dusheiko G, Bertoletti A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol. 2004;78:5707–5719. doi: 10.1128/JVI.78.11.5707-5719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, Alfieri A, Pesci M, Gaeta GB, Brancaccio G, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963–73.e9. doi: 10.1053/j.gastro.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Park SH, Rehermann B. Immune responses to HCV and other hepatitis viruses. Immunity. 2014;40:13–24. doi: 10.1016/j.immuni.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bengsch B, Spangenberg HC, Kersting N, Neumann-Haefelin C, Panther E, von Weizsäcker F, Blum HE, Pircher H, Thimme R. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J Virol. 2007;81:945–953. doi: 10.1128/JVI.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, Thimme R. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol. 2006;80:3532–3540. doi: 10.1128/JVI.80.7.3532-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernardin F, Tobler L, Walsh I, Williams JD, Busch M, Delwart E. Clearance of hepatitis C virus RNA from the peripheral blood mononuclear cells of blood donors who spontaneously or therapeutically control their plasma viremia. Hepatology. 2008;47:1446–1452. doi: 10.1002/hep.22184. [DOI] [PubMed] [Google Scholar]
- 30.Maylin S, Martinot-Peignoux M, Moucari R, Boyer N, Ripault MP, Cazals-Hatem D, Giuily N, Castelnau C, Cardoso AC, Asselah T, et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology. 2008;135:821–829. doi: 10.1053/j.gastro.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 31.Pham TN, MacParland SA, Mulrooney PM, Cooksley H, Naoumov NV, Michalak TI. Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J Virol. 2004;78:5867–5874. doi: 10.1128/JVI.78.11.5867-5874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radkowski M, Gallegos-Orozco JF, Jablonska J, Colby TV, Walewska-Zielecka B, Kubicka J, Wilkinson J, Adair D, Rakela J, Laskus T. Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology. 2005;41:106–114. doi: 10.1002/hep.20518. [DOI] [PubMed] [Google Scholar]
- 33.Dahari H, Feliu A, Garcia-Retortillo M, Forns X, Neumann AU. Second hepatitis C replication compartment indicated by viral dynamics during liver transplantation. J Hepatol. 2005;42:491–498. doi: 10.1016/j.jhep.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Castillo I, Rodríguez-Iñigo E, López-Alcorocho JM, Pardo M, Bartolomé J, Carreño V. Hepatitis C virus replicates in the liver of patients who have a sustained response to antiviral treatment. Clin Infect Dis. 2006;43:1277–1283. doi: 10.1086/508198. [DOI] [PubMed] [Google Scholar]
- 35.Hoare M, Gelson WT, Rushbrook SM, Curran MD, Woodall T, Coleman N, Davies SE, Alexander GJ. Histological changes in HCV antibody-positive, HCV RNA-negative subjects suggest persistent virus infection. Hepatology. 2008;48:1737–1745. doi: 10.1002/hep.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veerapu NS, Raghuraman S, Liang TJ, Heller T, Rehermann B. Sporadic reappearance of minute amounts of hepatitis C virus RNA after successful therapy stimulates cellular immune responses. Gastroenterology. 2011;140:676–685.e1. doi: 10.1053/j.gastro.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veerapu NS, Park SH, Tully DC, Allen TM, Rehermann B. Trace amounts of sporadically reappearing HCV RNA can cause infection. J Clin Invest. 2014;124:3469–3478. doi: 10.1172/JCI73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee WM, Polson JE, Carney DS, Sahin B, Gale M. Reemergence of hepatitis C virus after 8.5 years in a patient with hypogammaglobulinemia: evidence for an occult viral reservoir. J Infect Dis. 2005;192:1088–1092. doi: 10.1086/432917. [DOI] [PubMed] [Google Scholar]
- 39.Lin A, Thadareddy A, Goldstein MJ, Lake-Bakaar G. Immune suppression leading to hepatitis C virus re-emergence after sustained virological response. J Med Virol. 2008;80:1720–1722. doi: 10.1002/jmv.21257. [DOI] [PubMed] [Google Scholar]
- 40.Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014;11:209–219. doi: 10.1038/nrgastro.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Margeridon-Thermet S, Le Pogam S, Li L, Liu TF, Shulman N, Shafer RW, Najera I. Similar prevalence of low-abundance drug-resistant variants in treatment-naive patients with genotype 1a and 1b hepatitis C virus infections as determined by ultradeep pyrosequencing. PLoS One. 2014;9:e105569. doi: 10.1371/journal.pone.0105569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paolucci S, Fiorina L, Mariani B, Gulminetti R, Novati S, Barbarini G, Bruno R, Baldanti F. Naturally occurring resistance mutations to inhibitors of HCV NS5A region and NS5B polymerase in DAA treatment-naïve patients. Virol J. 2013;10:355. doi: 10.1186/1743-422X-10-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Applegate TL, Gaudieri S, Plauzolles A, Chopra A, Grebely J, Lucas M, Hellard M, Luciani F, Dore GJ, Matthews GV. Naturally occurring dominant drug resistance mutations occur infrequently in the setting of recently acquired hepatitis C. Antivir Ther. 2014:Epub ahead of print. doi: 10.3851/IMP2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Guillou-Guillemette H, Vallet S, Gaudy-Graffin C, Payan C, Pivert A, Goudeau A, Lunel-Fabiani F. Genetic diversity of the hepatitis C virus: impact and issues in the antiviral therapy. World J Gastroenterol. 2007;13:2416–2426. doi: 10.3748/wjg.v13.i17.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverman RH. Viral encounters with 2’,5’-oligoadenylate synthetase and RNase L during the interferon antiviral response. J Virol. 2007;81:12720–12729. doi: 10.1128/JVI.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haller O, Staeheli P, Kochs G. Interferon-induced Mx proteins in antiviral host defense. Biochimie. 2007;89:812–818. doi: 10.1016/j.biochi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 48.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 49.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 50.Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 51.Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, Tough DF. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 52.Gerlach N, Gibbert K, Alter C, Nair S, Zelinskyy G, James CM, Dittmer U. Anti-retroviral effects of type I IFN subtypes in vivo. Eur J Immunol. 2009;39:136–146. doi: 10.1002/eji.200838311. [DOI] [PubMed] [Google Scholar]
- 53.Gibbert K, Joedicke JJ, Meryk A, Trilling M, Francois S, Duppach J, Kraft A, Lang KS, Dittmer U. Interferon-alpha subtype 11 activates NK cells and enables control of retroviral infection. PLoS Pathog. 2012;8:e1002868. doi: 10.1371/journal.ppat.1002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hermann P, Rubio M, Nakajima T, Delespesse G, Sarfati M. IFN-alpha priming of human monocytes differentially regulates gram-positive and gram-negative bacteria-induced IL-10 release and selectively enhances IL-12p70, CD80, and MHC class I expression. J Immunol. 1998;161:2011–2018. [PubMed] [Google Scholar]
- 55.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 56.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 57.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 58.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 59.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 60.Larrubia JR, Lokhande MU, Moreno-Cubero E, García-Garzón S, Miquel J, Parra-Cid T, González-Praetorious A, Perna C, Lázaro A, Sanz-de-Villalobos E. HCV-specific CD8+ cell detection at week 12 of chronic hepatitis C treatment with PEG-interferon-α2b/ribavirin correlates with infection resolution. Cell Immunol. 2013;286:31–38. doi: 10.1016/j.cellimm.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Ahlenstiel G, Edlich B, Hogdal LJ, Rotman Y, Noureddin M, Feld JJ, Holz LE, Titerence RH, Liang TJ, Rehermann B. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231–129, 1231-129. doi: 10.1053/j.gastro.2011.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stegmann KA, Björkström NK, Veber H, Ciesek S, Riese P, Wiegand J, Hadem J, Suneetha PV, Jaroszewicz J, Wang C, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–1897. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 63.Guedj J, Rong L, Dahari H, Perelson AS. A perspective on modelling hepatitis C virus infection. J Viral Hepat. 2010;17:825–833. doi: 10.1111/j.1365-2893.2010.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dahari H, Shudo E, Ribeiro RM, Perelson AS. Mathematical modeling of HCV infection and treatment. Methods Mol Biol. 2009;510:439–453. doi: 10.1007/978-1-59745-394-3_33. [DOI] [PubMed] [Google Scholar]
- 65.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 66.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annu Rev Immunol. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- 68.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 69.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang X, Chen Y, Peng H, Tian Z. Memory NK cells: why do they reside in the liver? Cell Mol Immunol. 2013;10:196–201. doi: 10.1038/cmi.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 72.Abdel-Hakeem MS, Bédard N, Badr G, Ostrowski M, Sékaly RP, Bruneau J, Willems B, Heathcote EJ, Shoukry NH. Comparison of immune restoration in early versus late alpha interferon therapy against hepatitis C virus. J Virol. 2010;84:10429–10435. doi: 10.1128/JVI.01094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Badr G, Bédard N, Abdel-Hakeem MS, Trautmann L, Willems B, Villeneuve JP, Haddad EK, Sékaly RP, Bruneau J, Shoukry NH. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J Virol. 2008;82:10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamal SM, Fehr J, Roesler B, Peters T, Rasenack JW. Peginterferon alone or with ribavirin enhances HCV-specific CD4 T-helper 1 responses in patients with chronic hepatitis C. Gastroenterology. 2002;123:1070–1083. doi: 10.1053/gast.2002.36045. [DOI] [PubMed] [Google Scholar]
- 75.Morishima C, Musey L, Elizaga M, Gaba K, Allison M, Carithers RL, Gretch DR, McElrath MJ. Hepatitis C virus-specific cytolytic T cell responses after antiviral therapy. Clin Immunol. 2003;108:211–220. doi: 10.1016/s1521-6616(03)00142-6. [DOI] [PubMed] [Google Scholar]
- 76.Vertuani S, Bazzaro M, Gualandi G, Micheletti F, Marastoni M, Fortini C, Canella A, Marino M, Tomatis R, Traniello S, et al. Effect of interferon-alpha therapy on epitope-specific cytotoxic T lymphocyte responses in hepatitis C virus-infected individuals. Eur J Immunol. 2002;32:144–154. doi: 10.1002/1521-4141(200201)32:1<144::AID-IMMU144>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 77.Caetano J, Martinho A, Paiva A, Pais B, Valente C, Luxo C. Differences in hepatitis C virus (HCV)-specific CD8 T-cell phenotype during pegylated alpha interferon and ribavirin treatment are related to response to antiviral therapy in patients chronically infected with HCV. J Virol. 2008;82:7567–7577. doi: 10.1128/JVI.02175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tatsumi T, Takehara T, Miyagi T, Nakazuru S, Mita E, Kanto T, Hiramatsu N, Hayashi N. Hepatitis C virus-specific CD8+ T cell frequencies are associated with the responses of pegylated interferon-α and ribavirin combination therapy in patients with chronic hepatitis C virus infection. Hepatol Res. 2011;41:30–38. doi: 10.1111/j.1872-034X.2010.00734.x. [DOI] [PubMed] [Google Scholar]
- 79.Barnes E, Harcourt G, Brown D, Lucas M, Phillips R, Dusheiko G, Klenerman P. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology. 2002;36:743–754. doi: 10.1053/jhep.2002.35344. [DOI] [PubMed] [Google Scholar]
- 80.Zeuzem S, Pawlotsky JM, Lukasiewicz E, von Wagner M, Goulis I, Lurie Y, Gianfranco E, Vrolijk JM, Esteban JI, Hezode C, et al. International, multicenter, randomized, controlled study comparing dynamically individualized versus standard treatment in patients with chronic hepatitis C. J Hepatol. 2005;43:250–257. doi: 10.1016/j.jhep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 81.Pilli M, Zerbini A, Penna A, Orlandini A, Lukasiewicz E, Pawlotsky JM, Zeuzem S, Schalm SW, von Wagner M, Germanidis G, et al. HCV-specific T-cell response in relation to viral kinetics and treatment outcome (DITTO-HCV project) Gastroenterology. 2007;133:1132–1143. doi: 10.1053/j.gastro.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 82.Humphreys IS, von Delft A, Brown A, Hibbert L, Collier JD, Foster GR, Rahman M, Christian A, Klenerman P, Barnes E. HCV genotype-3a T cell immunity: specificity, function and impact of therapy. Gut. 2012;61:1589–1599. doi: 10.1136/gutjnl-2011-300650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barnes E, Gelderblom HC, Humphreys I, Semmo N, Reesink HW, Beld MG, van Lier RA, Klenerman P. Cellular immune responses during high-dose interferon-alpha induction therapy for hepatitis C virus infection. J Infect Dis. 2009;199:819–828. doi: 10.1086/597072. [DOI] [PubMed] [Google Scholar]
- 84.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Golden-Mason L, Klarquist J, Wahed AS, Rosen HR. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. J Immunol. 2008;180:3637–3641. doi: 10.4049/jimmunol.180.6.3637. [DOI] [PubMed] [Google Scholar]
- 86.Seigel B, Bengsch B, Lohmann V, Bartenschlager R, Blum HE, Thimme R. Factors that determine the antiviral efficacy of HCV-specific CD8(+) T cells ex vivo. Gastroenterology. 2013;144:426–436. doi: 10.1053/j.gastro.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 87.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim do Y, Ahn SH, Han KH. Emerging therapies for hepatitis C. Gut Liver. 2014;8:471–479. doi: 10.5009/gnl14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin B, Hennecke N, Lohmann V, Kayser A, Neumann-Haefelin C, Kukolj G, Böcher WO, Thimme R. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J Hepatol. 2014;61:538–543. doi: 10.1016/j.jhep.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 90.Odorizzi PM, Wherry EJ. Immunology. An interferon paradox. Science. 2013;340:155–156. doi: 10.1126/science.1237568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cooksley H, Chokshi S, Maayan Y, Wedemeyer H, Andreone P, Gilson R, Warnes T, Paganin S, Zoulim F, Frederick D, et al. Hepatitis B virus e antigen loss during adefovir dipivoxil therapy is associated with enhanced virus-specific CD4+ T-cell reactivity. Antimicrob Agents Chemother. 2008;52:312–320. doi: 10.1128/AAC.00467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmed F. What about us? Recent advances in the treatment of chronic hepatitis C threaten to leave some parts of the world behind. J Viral Hepat. 2013;20:367–368. doi: 10.1111/j.1365-2893.2012.01613.x. [DOI] [PubMed] [Google Scholar]
- 93.Petta S, Craxì A. Therapeutic algorithms for chronic hepatitis C in the DAA era during the current economic crisis: whom to treat? How to treat? When to treat? BMC Infect Dis. 2012;12 Suppl 2:S3. doi: 10.1186/1471-2334-12-S2-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]


