Figure 3.
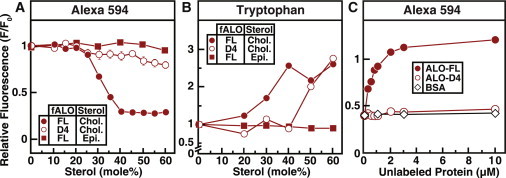
Interaction of fluorescently labeled ALO with sterol-containing membranes. Recombinant ALO-FL and ALO-D4 were overexpressed and purified, and fluorescently labeled versions (fALO-FL and fALO-D4) were generated as described in Materials and Methods. (A and C) Alexa 594 fluorescence of the labeled proteins. Reaction mixtures, in a final volume of 120 μL buffer B, contained 0.5 μM of the indicated fluorescently labeled protein and 67 μM liposomes comprised of DOPC and varying amounts of cholesterol (Chol.) or epicholesterol (Epi.) (A) or 0.5 μM fALO-FL, 67 μM liposomes comprised of 50 mol % DOPC and 50 mol % cholesterol, and varying amounts of the indicated unlabeled protein (C). (B) Intrinsic tryptophan fluorescence of the labeled proteins. Reaction mixtures, in a volume of 100 μL buffer B, contained 3.6 μM of the indicated protein and 600 μM liposomes composed of DOPC and varying mole fractions of cholesterol or epicholesterol. After incubation for 1 h at room temperature, Alexa Fluor 594 fluorescence (A and C) (excitation wavelength, 590 nm; emission wavelength, 617 nm; band pass, 2.5 nm for each) or intrinsic tryptophan fluorescence (B) (excitation wavelength, 290 nm; emission wavelength, 340 nm) was measured. For each protein, fluorescence from mixtures of protein with liposomes containing 0% cholesterol is normalized to 1.
