Figure 5.
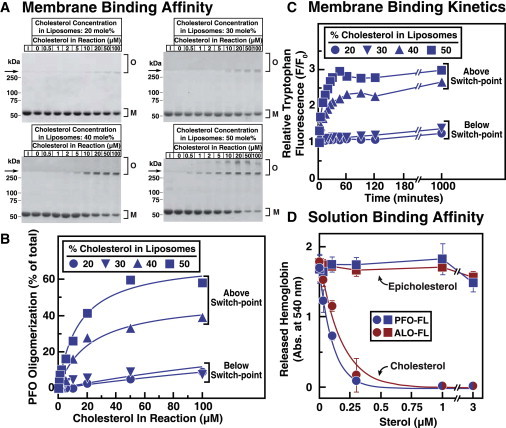
Affinity of PFO and ALO for cholesterol is determined by membrane phospholipids. Recombinant PFO-FL and ALO-FL were overexpressed and purified as described in Materials and Methods. (A and B) Affinity of PFO-FL for membrane cholesterol. Each reaction mixture, in a total volume of 1 mL of buffer B, contained 100 nM PFO-FL (5.7 μg) and varying amounts of liposomes composed of DOPC and the indicated amounts of cholesterol. (A) After incubation for 2 h at room temperature, PFO-FL was concentrated to a volume of 20 μL as described in Materials and Methods, and the entire amount of protein was subjected to SDS-PAGE. Lane 1 (I) in each gel contains 5.7 μg of PFO-FL (input amount) as a reference to judge the efficiency of PFO-FL concentration by Ni beads. Proteins were visualized with Coomassie Brilliant Blue R-250 stain. The molecular masses of protein standards are shown. Arrows indicate the interface between stacking and resolving gels. O, membrane-bound oligomeric form of PFO; M, free monomer form of PFO. (B) Gels were scanned and densitometric analysis was carried out to determine the percentage of the oligomeric, membrane-bound form of PFO relative to the total (membrane-bound oligomer plus free monomer). (C) Binding kinetics. Each reaction mixture, in a total volume of 200 μL of buffer B, contained 4.4 μM of PFO-FL and 600 μM liposomes composed of DOPC and the indicated amounts of cholesterol. After incubation at room temperature for the indicated times, intrinsic tryptophan fluorescence from the samples was measured (excitation wavelength, 290 nm; emission wavelength, 340 nm). The fluorescence from mixtures of PFO-FL with liposomes containing 0% sterol is normalized to 1. (D) Binding of DMSO-solubilized sterols to PFO-FL and ALO-FL. Each reaction mixture, in a final volume of 50 μL, contained either ALO-FL (1 nM) or PFO-FL (3 nM), and varying amounts of cholesterol or epicholesterol dissolved in DMSO (4% (v/v) final concentration). After incubation for 1 h at room temperature, 450 μL of rabbit erythrocytes (washed and diluted as described in Materials and Methods) was added to each reaction mixture. After incubation for 10 min at 37°C, the extent of hemolysis was quantified by measuring the release of hemoglobin (absorbance at 540 nm).
