Figure 1.
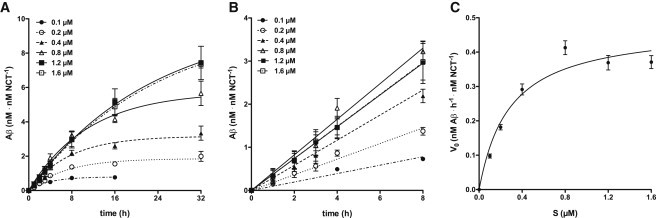
In vitro kinetics of γ-secretase. (A) Time course of in vitro Aβ generation by γ-secretase. γ-Secretase proteoliposomes were incubated with 0.1, 0.2, 0.4, 0.8, 1.2, and 1.6 μM of C100-His6 APP substrate, respectively, at 37°C. For each indicated incubation time (t = 0, 1, 2, 3, 4, 8, 16, and 32 h), the respective concentration of total Aβ was measured. The data points represent the mean ± SE of two to five independent experiments (see Materials and Methods). Note that for some data points the error bars are too small to be displayed. The lines represent fitted curves to single exponential association kinetics. (B) Data from A for the first 8 h of incubation. For each substrate concentration, V0 was obtained from the slope at t = 0 by fitting the data to a linear increase of Aβ during time. (C) Michaelis-Menten plot. For each individual substrate concentration (S), the fitted mean ± SE of V0 from the data in (B) was taken. The line represents the fit of the data points to the Michaelis-Menten equation. From this fit, the values for Vmax (0.476 ± 0.047 nM Aβ h−1 nM NCT−1) and (0.285 ± 0.091 μM) were obtained.
