Figure 2.
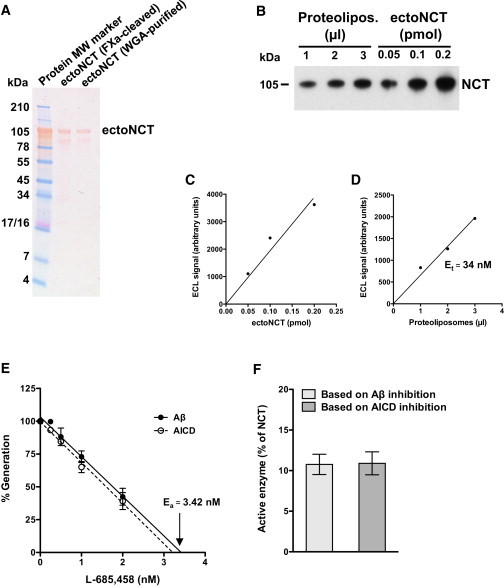
Quantification of γ-secretase in the in vitro assay. (A) SDS-PAGE analysis of the recombinant ectoNCT. Ponceau S staining showed that both ectoNCT preparations yielded purified protein that migrated at the expected MW. A higher degree of purity was obtained for the WGA-purified ectoNCT, qualifying this protein preparation as standard for the γ-secretase determination. (B) Immunoblot analysis of γ-secretase proteoliposomes in comparison with the ectoNCT protein standard. Small aliquots of one of the proteoliposome preparations used for the in vitro assays shown in Fig. 1 A were comigrated with known amounts of purified ectoNCT protein standard and detected with an anti-NCT monoclonal antibody. (C and D) Determination of total γ-secretase (Et). (C) The ECL signal intensities of the immunoblot shown in (B) were plotted to obtain an ectoNCT standard calibration curve. (D) From the same immunoblot, the ECL signal intensities of aliquots of the proteoliposomes were compared with the calibration curve, allowing for an estimation of the concentration of NCT, i.e., the total γ-secretase present in the proteoliposomes. Et was 34 nM. (E) Active-site titration of γ-secretase. There was a close correlation between the decrease in Aβ and AICD production with increasing L-685,458 concentrations. Data points represent the mean ± SE of three measurements. Ea was 3.42 nM or 3.21 nM based on the extrapolation of Aβ and AICD data, respectively. (F) Determination of the active γ-secretase enzyme pool. Et, determined from the amount of NCT (D) was compared with Ea, determined from the active site inhibitor titration (E). The fraction of active γ-secretase in proteoliposomes (Ea/Et) was 10.1% and 9.5% based on the Aβ and AICD data (E), respectively. In a total of four independent enzyme purifications, we found that only 11% (mean ± SE = 10.8 ± 1.2) of γ-secretase present in the proteoliposomes was in an active conformation.
