Abstract
How infectious disease agents interact with their host changes during the course of infection and can alter the expression of disease-related traits. Yet by measuring parasite life-history traits at one or few moments during infection, studies have overlooked the impact of variable parasite growth trajectories on disease evolution. Here we show that infection-age-specific estimates of host and parasite fitness components can reveal new insight into the evolution of parasites. We do so by characterizing the within-host dynamics over an entire infection period for five genotypes of the castrating bacterial parasite Pasteuria ramosa infecting the crustacean Daphnia magna. Our results reveal that genetic variation for parasite-induced gigantism, host castration and parasite spore loads increases with the age of infection. Driving these patterns appears to be variation in how well the parasite maintains control of host reproduction late in the infection process. We discuss the evolutionary consequences of this finding with regard to natural selection acting on different ages of infection and the mechanism underlying the maintenance of castration efficiency. Our results highlight how elucidating within-host dynamics can shed light on the selective forces that shape infection strategies and the evolution of virulence.
Keywords: Daphnia, Pasteuria ramosa, life history, genetic variation, host–parasite interactions
1. Introduction
The evolution of parasite life history has traditionally been studied by characterizing components of host and parasite fitness at one or few times during the course of infection [1–4]. This approach has proved appropriate for understanding how complex interactions between host and parasite genotypes, as well as environment factors, influence the outcome of infection. Studying host and parasite life histories in such a way has led to major insights into why parasites induce specific levels of host damage and mortality [5,6] or why some strains are of moderate severity for their hosts, whereas others provoke serious harm [7]. Yet characterizing host–parasite interactions via single snapshots overlooks the fact that the growth trajectory of a parasite during infection is an evolvable trait itself [8,9] and that host exploitation may vary considerably over the course of an infection.
Recent theoretical work has reinforced how an understanding of infection-age-specific changes in transmission and virulence can inform subsequent between-host processes and pathogen evolution [9,10]. Mideo et al. [11] showed that levels of genetic variation at particular time points of infection determine the trade-offs between early and late transmission in rodent malaria, and in turn impact the outcome of between-host selection. By focusing on changes in genetic variation arising from within-host dynamics, their approach provided an alternative to the need for a detailed understanding of within-host processes to study parasite evolution. However, datasets covering a sufficient amount of natural genetic variation among parasite genotypes are required to apply to such models, which to date are scarce (see discussion in [10]). Assessing how host and parasite life-history traits change in response to each other over the course of an infection is the first step to discover the critical phases in the infection process that influence disease evolution.
In this study, we explored how an understanding of within-host dynamics can shed light on the potential selective forces that shape infection strategies and the evolution of virulence. To do so, we used the crustacean water flea Daphnia magna and its castrating bacterial parasite Pasteuria ramosa. The trait ‘castration’—parasite-induced suppression of host reproduction—is common in many parasite taxa [12–14]. It has evolved as a parasite strategy to overcome the virulence–transmission trade-off, as exploitation of the host's reproductive resources does not interfere with host survival [15]. The amount of energy an adult female typically allocates to reproduction is high, making the reproductive tissue a rich exploitable resource that is available throughout the host's life [14]. Thus, the strength and duration of castration should be linked to the parasite's reproductive success. However, empirical data on these key components of castration are lacking.
In Daphnia, host castration by P. ramosa can be reversed with the use of antibiotics [16], suggesting that the parasite must continually maintain control over host reproduction via chemical or hormonal means. The control of host reproduction, however, is not always perfect. Mageroy et al. [17] showed that imperfect castration reduces parasite transmission, spore production and increases host lifetime reproductive success. Hall & Ebert [18] suggested that variability in castration might cause disease dynamics to change with infection age, as they found differences in genetic variation for parasite fitness at early and late stages of the infection process. Indeed, in this system, host and parasite traits like virulence (parasite-induced mortality and fecundity reduction) and resistance show strong G × G and G × E interactions (G, genetic; E, environmental) [3,19–22], which suggest a genetic basis for variation in pathology caused by P. ramosa. Unravelling how variability in castration dynamics is linked to overall within-host dynamics will help identify how conflict between host and parasites over resources can be resolved.
We evaluated the dynamics of the within-host process using five parasite genotypes in two separate experiments. In the first experiment, infected hosts were destructively sampled and characteristics of disease quantified at short time intervals over a period of 51 days. In a second experiment, we followed hosts until parasite-induced death and measured total lifetime parasite fitness. The traits monitored were reduction in host fecundity (virulence measured as parasite-induced castration), host size (parasite-induced gigantism) and parasite transmission stage production (parasite fitness). We addressed the following questions. (i) How do characteristics of disease change over the course of an entire infection period? (ii) Are differences among parasite genotypes structured over time? (iii) What are the potential consequences of temporally structured genetic variation for pathogen evolution?
2. Material and methods
(a). Daphnia–Pasteuria study system
Daphnia magna Straus lives in a diverse array of freshwater habitats, from lakes to rock-pools. It acquires algal food through filter feeding and reproduction takes place by cyclic parthenogenesis. Pasteuria ramosa Metchnikoff 1888 is a gram-positive bacterial parasite of Daphnia, found throughout the Northern Hemisphere [23]. Infection takes place after the uptake of parasite spores by filter feeding. After successful invasion, the parasite proliferates to fill the entire body cavity with spores, and host reproduction ceases shortly after infection (castration). Transmission occurs exclusively horizontally by the release of several millions of spores after death of infected hosts.
We made use of five P. ramosa genotypes with origins spanning a wide geographical range and ecological conditions: Pasteuria clone C1 (Russia), C14 (Finland), C19 (Germany), C20 (UK) and C24 (Belgium). These parasite lines have been derived from single spore infections (see Luijckx et al. [22]) and therefore represent true single-genotypes. The entire experiment was conducted using the host clone D. magna HO2, originally collected in Hungary and being chosen because it is susceptible to all P. ramosa genotypes included in our experiments.
(b). Experiment 1: infection-age-specific patterns of disease
To begin, 500 newly hatched offspring from synchronized mothers were randomly distributed across each of the five parasite treatments (100 replicates per treatment). We also established 28 control individuals that were not exposed to parasite spores. The infection protocol was as follows: hosts were raised individually in jars filled with 20 ml ADaM (artificial medium, [24]) for 3 days. On days 4 and 5, an infection dose of 20 000 parasite spores was added to each jar (40 000 spores total). Spore doses for each Pasteuria genotype were prepared by crushing a previously infected animal, counting the spore loads with a Neubauer improved counting chamber, and diluting the solution to the required concentration. On day 6, and every 3 days thereafter, hosts were transferred to fresh 100-ml jars filled with 80 ml ADaM. This day is counted as the first day post-exposure (DPE). All animals were kept in a single climate-chamber (16 L : 8 D, 20°C ± 0.5°C) and the shelf position was rearranged daily to avoid positional effects. Food was steadily increased to 8 million algal cells per individual per day (Scenedemus sp.) to account for the growing demands of the animals.
For each parasite genotype, we destructively sampled five to eight individuals every 3 days and recorded body size and parasite spore production. This began at 18 DPE, as counting of fully developed spores is unreliable before this time. Host body size was measured using a scaled binocular microscope, prior to freezing of the animals for later spore counting. At the same time, the number of offspring a host produced was recorded at every medium change. Thus, we can estimate host fecundity until sampling (the total number of offspring a female produced until any given day) and time-specific host fecundity (the number of offspring a female produced between two sampling time points). Because of high infection rates (range 85 to 97%), we continued sampling until 51 DPE, except for C20, where sampling ended at 48 DPE because of slightly higher host mortality. During the experiment, none of the control individuals became infected and mortality was minimal.
(c). Experiment 2: relationship between parasite transmission and virulence
The set-up and infection procedure for this experiment was the same as for experiment 1, but with 28 replicates per parasite treatment plus unexposed controls (168 individuals in total). We monitored hosts until parasite-induced death and measured total lifetime parasite fitness. Offspring were counted at each changing event (twice a week), with survival monitored daily and any dead animals frozen for later spore counting. At 18 DPE, hosts not showing any of the typical signs of infection (castration, gigantism, reddish-brown colour) were removed from the experiment.
(d). Statistical analysis
All statistical analyses were performed in R (v. 3.0.1; available at: www.R-project. org). For experiment 1, we excluded data from three individuals infected by parasite clone C20. They showed offspring counts similar to the controls, suggesting incorrect identification of infection status. For all following analyses, residual values were checked for normality and data for spore load and host fecundity were square-root transformed to meet normality. Linear regression was used to characterize the cumulative increase in measured traits over time (DPE). Differences in the regression coefficients between parasite genotypes were assessed based on the significance of the interaction between the predictor and parasite genotype. We also compared differences between pathogen genotypes at different age classes, by dividing the sampling period into four independent time intervals: interval 1 includes the measurements for 18–24 DPE, interval 2 for 27–33 DPE, interval 3 for 36–42 DPE and interval 4 for 45–51 DPE. At each time interval, mean trait values were compared using analysis of variance with pathogen genotype as a factor.
For experiment 2, we compared the difference in mean trait values between the parasite genotypes using a one-way factor analysis of variance, following the same general process as above. We then analysed the relationship between parasite and host fitness separately for each parasite genotype using multiple regression. Based on the outcome of the previous experiment, we subdivided fecundity data into early and late components based on the period between the two fecundity peaks at 18 DPE (figure 3). Before analysis we scaled parasite spore loads (our fitness measure) to a relative fitness measure by dividing by the mean within each genotype. We then standardized all other traits to a mean of zero and standard deviation of one to allow a direct comparison of the relative strengths of each trait of interest on parasite fitness. As above, partial F-tests were used to test for differences in the regression coefficients between parasite genotypes.
Figure 3.
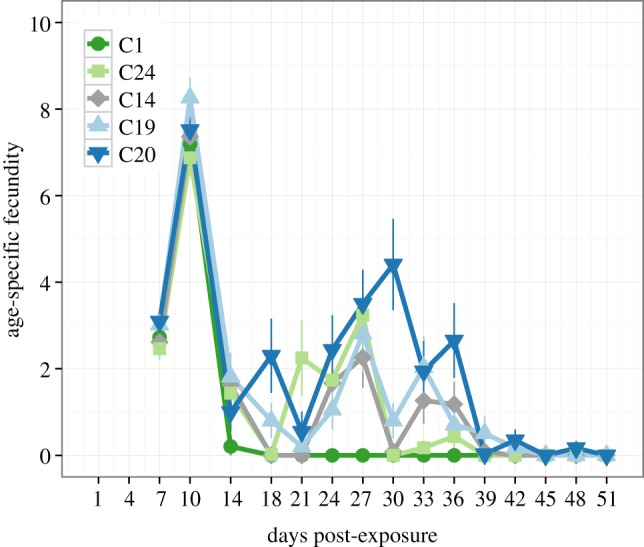
The infection-age-specific estimates of host fecundity for D. magna infected with five P. ramosa genotypes. The value at each time point represents the number of offspring produced within the previous 3-day period (e.g. a value of 4 at day 30 indicates that an animal sampled at that time point produced a clutch of four offspring sometime between days 28 and 30).
3. Results
(a). Infection-age-specific patterns of disease
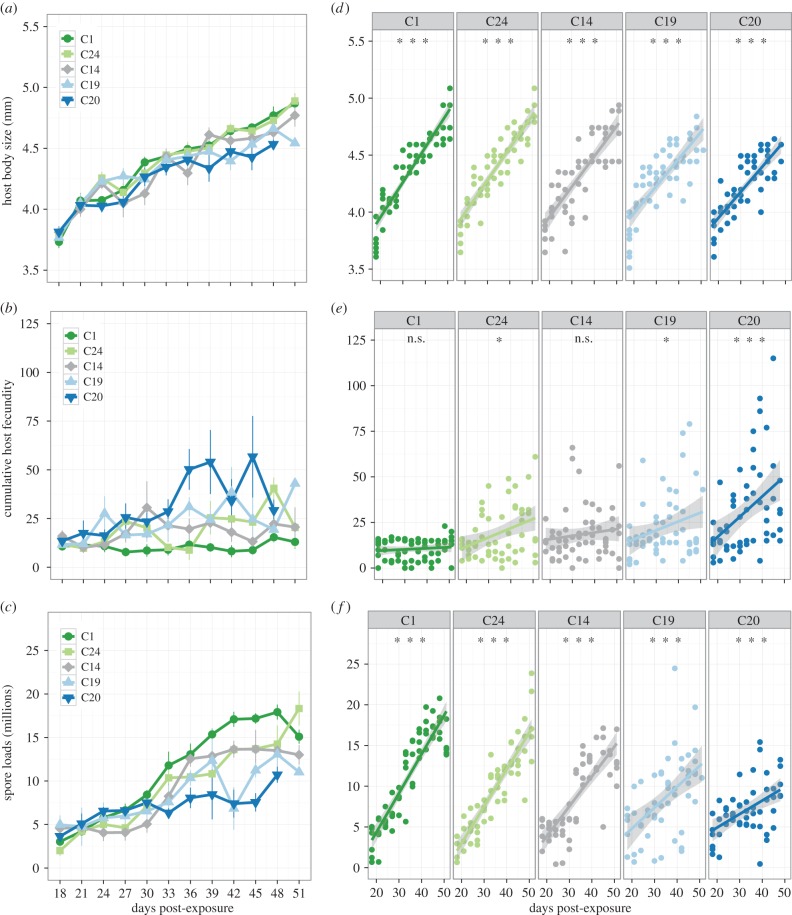
Our results reveal that parasite genotypes vary in how host body size, fecundity of infected hosts and spore loads change over an infection period of 51 days (12 sampling points, figure 1a–c). The increase in body size was relatively consistent between the parasite genotypes, as all infected hosts were approximately 12% larger than the controls at day 51 (control size: 4.2 mm ± 0.1 s.e.). Yet there were still subtle differences among genotypes (partial F-test: F4,284 = 2.40, p = 0.05). Figure 1a shows that the greatest increase in size occurred for C1 and the least increase for C20.
Figure 1.
Development of disease traits for five parasite genotypes sampled over a period of 51 DPE. (a–c) The average increase in body size, cumulative host fecundity and spore loads (in millions per infected female); (d–f) the underlying linear trends for each specific parasite genotype. As animals were sampled destructively to estimate spore loads, we maintained equal sample sizes at each time point by including only individuals' value for body size and cumulative fecundity at their specific time of sampling. (n.s. not significant p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001).
By contrast, the rate of increase in host fecundity and spore loads was clearly more variable among the parasites (figure 1b,c). In all cases, infected females had much lower total fecundity than controls (p < 0.01, control fecundity at day 51: 210.9 ± 4.6 s.e.), but the rate of increase in host fecundity differed significantly between the parasite genotypes (partial F-test: F4,287 = 3.11, p = 0.02). For example, the fecundity of hosts infected with parasite C1 did not increase after the initial bout of reproduction, whereas the cumulative fecundity of C20 infected females continued to rise (figure 1e). The increase in spore loads also differed with pathogen genotype (partial F-test: F4,278 = 8.10, p < 0.01), but the rank order of genotypes was opposite to that of fecundity. As shown in figure 1f, genotype C20 experienced the smallest increase over the study period, rising only to 10 million spores, while C1 increased to nearly 20 million spores at day 51.
For each of the three traits, the significant genotype by time interaction terms indicate that the progression of disease over time appears to be genotype specific. To explore these patterns further, we grouped trait values into four time intervals (figure 2). A separate analysis of variance for each of the four time intervals showed that differences among parasite genotypes were significant typically towards the end of the infection process (figure 2a–c), indicating an increase in genetic variation among parasite genotypes over time for these traits. Correspondingly, we see an increase in the amount of variation explained by parasite genotype, as estimated by the genotypic effect sizes for host size (4% at 18–24 days to 25% at 45–51 days), fecundity (5% at 18–24 days to 22% at 45–51 days) and spore loads (4% at 18–24 days to 40% at 45–51 days).
Figure 2.
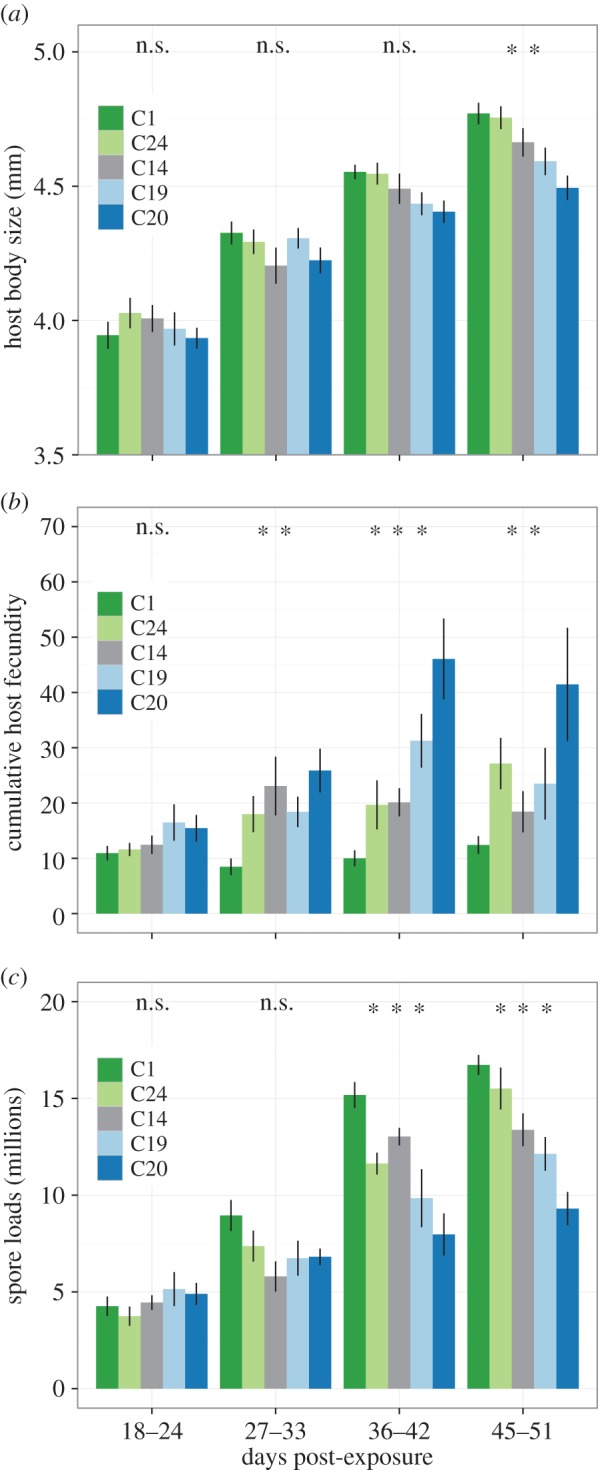
Age-class-specific averages for the cumulative estimates of (a) host body size, (b) cumulative host fecundity per infected female and (c) spore loads (in millions per infected female). Data are grouped into four time intervals. Significance differences among genotypes are indicated above each time interval (n.s., not significant; p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001).
(b). Relationship between parasite transmission and virulence
Comparing the last interval (45–51 DPE) in figure 2 shows that the host fecundity order of parasite genotypes is roughly the inverse of that for spore loads. Underlying the differences between the initial and the chronic phases of the infection appears to be the efficiency with which P. ramosa is able to maintain effective castration. This is illustrated in figure 3, where the number of offspring produced at any sampling time point (as opposed to cumulative fecundity in figure 1) is highly variable, especially 18–36 DPE. Hosts stay castrated when infected with parasite C1, but regain reproduction and overcome initial castration when infected with the other parasite clones (especially C20). This relief from castration is temporary with fecundity returning to near zero for all clones around day 39, approximately 2 weeks before the likelihood of parasite-induced host death increases.
To explore this relationship further, we conducted a second experiment where we monitored host and parasite fitness until host death (table 1). Consistent with the previous experiment in both the rank order of genotypes and mean trait values, we found that parasite genotypes differed in spore production, total fecundity and the time until host death. There was little variation in the onset of castration, as differences in host fecundity were only evident late in the infection process (i.e. castration efficiency, more than 18 days post-infection). The least efficient castrators again produced the fewest spores (C19 and C20).
Table 1.
Mean (and s.e.) trait values for infected animals in experiment 2, where animals were maintained until host death. Shown are the spore load of an infected host, the time until host death (infection duration), total fecundity, early fecundity and late fecundity, based on a cut-off of 18 days post-exposure (DPE).
| parasite genotype |
one-way ANOVA |
||||||
|---|---|---|---|---|---|---|---|
| trait | C1 (n = 24) | C24 (n = 27) | C14 (n = 18) | C19 (n = 24) | C20 (n = 27) | F4,113 | p-value |
| spore loads (millions) | 15.11 (0.99) | 18.45 (1.73) | 16.63 (1.60) | 8.56 (1.40) | 8.57 (0.96) | 11.57 | <0.001 |
| infection duration (days) | 50.25 (1.52) | 57.31 (1.79) | 57.94 (3.74) | 45.29 (1.60) | 46.96 (1.25) | 9.76 | <0.001 |
| total fecundity | 13.00 (1.17) | 20.96 (3.06) | 21.77 (5.66) | 48.08 (6.55) | 37.37 (4.60) | 10.92 | <0.001 |
| early fecundity (<18 DPE) | 12.50 (0.96) | 11.77 (0.85) | 10.44 (1.07) | 12.67 (0.69) | 13.63 (1.03) | 0.93 | 0.452 |
| late fecundity (≥18 DPE) | 0.50 (0.50) | 9.19 (2.79) | 12.69 (7.11) | 35.42 (6.69) | 24.59 (4.73) | 9.91 | <0.001 |
Using multiple regressions, we assessed how variation in the duration of infection and host fecundity impacted on parasite spore production. Overall, spore production increased with the duration of infection (β = 0.72 ± 0.06, p < 0.01) and decreased with total fecundity (β = −0.42 ± 0.08, p < 0.01). Yet, this pattern varied with parasite genotype (partial F-test: F12,97 = 2.00, p = 0.03) and whether early or late reproduction was considered (table 2). For all genotypes, the duration of infection was the dominant predictor of spore production (highest standardized regression coefficient) and was universally associated with strong increases in spore numbers. Genotypes differed only in the strength of the infection duration effect (genotype by duration interaction, p < 0.01), rather than any trend reversals. Late fecundity was negatively associated with spore numbers, although the strength of this trend was more varied—being an important predictor for C19 and C20 genotypes, marginal for C1 and not significant for C24 an C14. For all parasite genotypes, however, early fecundity, and thus the onset of castration, were unrelated to variation in spore production.
Table 2.
The standardized regression coefficients (and s.e.) for the impact of infection duration, early and late fecundity on parasite spore production. Each parasite genotype was analysed using a separate multiple regression (#< 0.1, *< 0.05, **< 0.01, ***< 0.001).
| parasite genotypes |
|||||
|---|---|---|---|---|---|
| regression variables | C1 | C24 | C14 | C19 | C20 |
| infection duration (days) | 0.61*** (0.09) | 0.83*** (0.16) | 0.72*** (0.13) | 0.96*** (0.14) | 0.70*** (0.13) |
| early fecundity (<18 DPE) | 0.13 (0.09) | −0.19 (0.16) | −0.21 (0.12) | −0.09 (0.15) | 0.02 (0.14) |
| late fecundity (≥18 DPE) | −0.17# (0.09) | −0.28 (0.20) | −0.40** (0.13) | −0.33* (0.15) | −0.29* (0.14) |
4. Discussion
Recent work has suggested that genetic variation in disease characteristics can vary considerably over the course of infection, potentially influencing the evolution of the parasite [9,11,18]. Here, we quantified parasite genetic variation for three key disease traits over the duration of an entire infection process. Our results show that the relative contribution of Pasteuria genotypes to disease traits is infection-age-specific and that variability late in the infection process has the largest impact on parasite fitness. We discuss our findings in terms of the infection-age-specific evolutionary potential of disease, the mechanisms underlying parasite fitness in P. ramosa and the potential for natural selection to act on parasite fitness.
(a). The evolutionary potential of disease can be infection-age-specific
The evolutionary potential of disease depends not only on the growth rate of an individual pathogen, but also when variability among genotypes is greatest. With malaria, for example, within-host growth is most pronounced early in infection [25,26], yet much of the genetic variability results from how pathogen loads are maintained later in the infection process [27]. In our study, P. ramosa grew continuously over the course of infection and differences among genotypes in virulence (reduced fecundity) and spore loads increased with time since exposure (figure 1). The relationship between transmission and virulence also changed, as only late in the infection process were virulence and transmission potential positively correlated. Thus, for the five parasite genotypes studied here, the evolutionary potential (i.e. the degree of differences among parasite genotypes) of virulence and transmission changes with the age of the infection, raising the question whether such within-host dynamics matter for the evolution of the parasite and thus for disease traits?
Ultimately, the consequences of any within-host patterns for evolutionary dynamics depend on a direct link to parasite transmission success [9,11,18]. A lack of variability (and evolutionary potential) early in the infection process would be irrelevant if all infected animals were able to progress through to the end of the infection process, when the parasite induces host death. However, this rarely reflects the situation in nature, where Daphnia frequently suffer from diverse sorts of extrinsic mortality, including hypoxia, food stress and predation [28,29]. In this context, it has already been shown that accelerated host death is ecologically and epidemiological important. Predation on infected animals, for example, has been shown to be a modifier of infection dynamics in Daphnia [29,30], as predators shortened the infection period by releasing spores from their infected prey during feeding (‘sloppy predator’ [31]), thereby selecting for accelerated parasite development [30]. Indeed, Pasteuria spores are infectious as early as 14 days post-infection ([32]; M. Clerc 2012, personal observation) and any extrinsic mortality from this moment onwards would allow transmission and alter evolutionary dynamics.
The assumption that extrinsic host mortality is an important factor for shaping parasite evolution is not new [5,33]. However, research on the impact of a shortened infection period has focused mainly on the reduction in parasite transmission potential, rather than on differences in the genetic architecture of disease traits that will vary across time (but see [30]). Our results suggest that by shortening host lifespan, any extrinsic mortality would not only alter overall parasite transmission rates but also would diminish differences in fitness among parasite genotypes and thus alter the evolutionary potential of the disease. These fitness costs will be most strongly felt by the fastest-growing parasite. A shortening of the infection process to 30 DPE, for example, will result in a greater loss of potential spores for parasite genotype C1 than C20 (figure 1). Such findings, therefore, highlight how infection-age-specific patterns of disease can be used to predict the impact of extrinsic factors on disease evolution.
(b). Within-host dynamics and insights into the biology of castration
Underlying the infection-age-specific patterns appear to be differences in how well the parasite controls host reproduction. Castration is a strategy of the parasite to increase resource availability and avoid host death via resource depletion. Models of its evolution suggest instantaneous and complete castration to be the optimal level of exploitation for the parasite [34,35]. By characterizing the within-host dynamics of castration, we show that castration is often not complete for Pasteuria. As shown in figure 3, certain genotypes appear to temporarily lose control over castration in the second half of the infection process (C20 and C19), whereas the most efficient castrator (C1) is able to completely suppress host reproduction from day 14 onwards. These results suggests that castration may have two components, one being the initial onset of castration (castration onset) and the other how well castration is maintained throughout the infection process (castration efficiency).
Together with Mageroy et al. [17] and Hall & Ebert [18], there is now clear evidence that castration is not perfect in this system. In all studies, hosts that regained reproductive capability contained fewer spores than completely castrated hosts, reinforcing the view that castration is adaptive for Pasteuria. Using data collected from individuals at death, however, we formally quantified the importance of castration onset (offspring produced before 18 DPE), castration efficiency (offspring produced after 18 DPE) and the duration of infection (days until host death). Our results reveal that the length of infection is the best predictor of parasite fitness (the longer—the better) and that any influence of host reproduction is limited to late in the infection process (table 2). This suggests that parasite genotypes only realize their full fitness potential by maintaining control of host reproduction throughout the entire infection process.
For the one host and five parasite genotypes used in this study, it appears that the relationship between host castration and parasite fitness [36] is driven by the maintenance of castration (castration efficiency), rather than the initial act of gaining control of host reproduction (castration onset). For other unmeasured host and parasite combinations, however, the relative importance of castration onset and efficiency will likely vary. A number of studies have documented differences between host and parasite genotypes in many characteristics of the early phase of infection, including time until initial castration, early fecundity and initial spore growth [18,21,36–38]. By using only one host genotype, we inevitably excluded an important component of variability, including the potential for host-by-pathogen genetic interactions. Our results nonetheless highlight the potential importance for infection-age-specific processes to shape parasite genetic variation in certain cases.
(c). What maintains genetic variation in castration efficiency?
If castration efficiency is strongly correlated with parasite fitness, why then do we observe differences among parasite genotypes in their ability to cause persistent castration? By contrast, the onset of castration, at least for the host–parasite combinations studied here, was uncorrelated with spore production and displayed little genetic variation. From a mechanistic perspective, it is assumed that castration is achieved by chemical or molecular interference, and not the destruction of reproductive tissue, as it can be reversed by curing an infection with antibiotics [16]. Castration efficiency, therefore, may depend on the ability of a parasite to constantly maintain certain developmental stages within the host—in particular growing parasite cells, but not inert resting spore stages—or go through several rounds of spore development. This physiological model of castration efficiency, however, would not explain why variability in castration efficiency should be maintained, as selection is expected to maximize castration efficiency.
We suggest three hypotheses for why a seemingly disadvantageous trait such as castration inefficiency would persist. First, as Daphnia are typically infected by multiple Pasteuria genotypes [39], it is possible joint action between multiple genotypes that helps to maintain castration. As long as one genotype is maintaining control of castration, then another genotype's inefficiency is never exposed. This line of argument would explain why earlier studies using Pasteuria isolates, which contain multiple genotypes, rarely observed incomplete castration (e.g. [36]). Second, ineffective castrators might be maintained because of benefits received earlier in the infection process. Castration efficiency, for example, may trade-off with other fitness components such as the production of robust spores or immune evasion. Finally, inefficient castration may well be detrimental, but the trait is never exposed to natural selection. If Daphnia in the wild suffer extrinsic mortality before any fitness loss even occurs (i.e. before day 30), then selection on late-acting traits such as castration efficiency will be much weaker, preventing the removal of genotypes that lose control of host reproduction.
In summary, our results highlight how elucidating within-host dynamics over the course of an infection can generate new insights into pathogen evolution. Unravelling the potential contributions of these evolutionary mechanisms, however, will require characterizing the course of infection for parasite genotypes from populations with differences in Daphnia survival [30]. Although all the parasite clones used in this study were derived from natural isolates, we know little about their natural history (but see [22]). Given the diverse geographical distribution of the parasite origins, each population would have likely varied in the permanence of the population (seasonal or permanent) and the type of predators (fish, invertebrate or none)—all of which would shape the duration of an infection period. Ecological information such as this will be key for dissecting the importance of either weakened selection or joint action between efficient and inefficient castrators as an explanation for the evolution of an apparently disadvantageous trait such as castration inefficiency.
Supplementary Material
Supplementary Material
Acknowledgements
We thank A. Roulin and C. Metzger for comments on the manuscript and J. Hottinger and U. Stiefel for laboratory assistance.
Funding statement
This study was supported by an EU Marie Curie Incoming International Fellowship (PIIF-GA-2009-252417) to M.D.H. and by the Swiss National Science Foundation.
References
- 1.Bedhomme S, Agnew P, Sidobre C, Michalakis Y. 2004. Virulence reaction norms across a food gradient. Proc. R. Soc. Lond. B 271, 739–744. ( 10.1098/rspb.2003.2657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fellous S, Koella JC. 2010. Cost of co-infection controlled by infectious dose combinations and food availability. Oecologia 162, 935–940. ( 10.1007/s00442-009-1535-2) [DOI] [PubMed] [Google Scholar]
- 3.Vale PF, Stjernman M, Little TJ. 2008. Temperature-dependent costs of parasitism and maintenance of polymorphism under genotype-by-environment interactions. J. Evol. Biol. 21, 1418–1427. ( 10.1111/j.1420-9101.2008.01555.x) [DOI] [PubMed] [Google Scholar]
- 4.Salvaudon L, Héraudet V, Shykoff JA, Hraudet V. 2005. Parasite–host fitness trade-offs change with parasite identity: genotype-specific interactions in a plant–pathogen system. Evolution 59, 2518–2524. ( 10.1111/j.0014-3820.2005.tb00965.x) [DOI] [PubMed] [Google Scholar]
- 5.Anderson RM, May RM. 1982. Coevolution of hosts and parasites. Parasitology 85, 411–426. ( 10.1017/S0031182000055360) [DOI] [PubMed] [Google Scholar]
- 6.Bérénos C, Wegner KM, Schmid-Hempel P. 2010. Antagonistic coevolution with parasites maintains host genetic diversity: an experimental test. Proc. R. Soc. B 278, 218–224. ( 10.1098/rspb.2010.1211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson HM, Mackinnon MJ, Chan BH, Read AF. 2003. Mosquito mortality and the evolution of malaria virulence. Evolution 57, 2792–2804. ( 10.1111/j.0014-3820.2003.tb01521.x) [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick M, Lofsvold D, Bulmer M. 1990. Analysis of the inheritance, selection and evolution of growth trajectories. Genetics 124, 979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day T, Alizon S, Mideo N. 2011. Bridging scales in the evolution of infectious disease life histories: theory. Evolution 65, 3448–3461. ( 10.1111/j.1558-5646.2011.01394.x) [DOI] [PubMed] [Google Scholar]
- 10.Mideo N, Alizon S, Day T. 2008. Linking within- and between-host dynamics in the evolutionary epidemiology of infectious diseases. Trends Ecol. Evol. 23, 511–517. ( 10.1016/j.tree.2008.05.009) [DOI] [PubMed] [Google Scholar]
- 11.Mideo N, Nelson WA, Reece SE, Bell AS, Read AF, Day T. 2011. Bridging scales in the evolution of infectious disease life histories: application. Evolution 65, 3298–3310. ( 10.1111/j.1558-5646.2011.01382.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clay K. 1991. Parasitic castration of plants by fungi. Trends Ecol. Evol. 6, 162–166. ( 10.1016/0169-5347(91)90058-6) [DOI] [PubMed] [Google Scholar]
- 13.Jokela J, Lively CM, Taskinen J, Peters AD. 1999. Effect of starvation on parasite-induced mortality in a freshwater snail (Potamopyrgus antipodarum). Oecologia 119, 320–325. ( 10.1007/s004420050792) [DOI] [PubMed] [Google Scholar]
- 14.Lafferty KD, Kuris AM. 2009. Parasitic castration: the evolution and ecology of body snatchers. Trends Parasitol. 25, 564–572. ( 10.1016/j.pt.2009.09.003) [DOI] [PubMed] [Google Scholar]
- 15.Baudoin M. 1975. Host castration as a parasitic strategy. Evolution 29, 335–352. ( 10.2307/2407221) [DOI] [PubMed] [Google Scholar]
- 16.Little TJ, Ebert D. 2000. The cause of parasitic infection in natural populations of Daphnia (Crustacea: Cladocera): the role of host genetics. Proc. R. Soc. Lond. B 267, 2037–2042. ( 10.1098/rspb.2000.1246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mageroy JH, Grepperud EJ, Jensen KH. 2011. Who benefits from reduced reproduction in parasitized hosts? An experimental test using the Pasteuria ramosa–Daphnia magna system. Parasitology 138, 1910–1915. ( 10.1017/S0031182011001302) [DOI] [PubMed] [Google Scholar]
- 18.Hall MD, Ebert D. 2012. Disentangling the influence of parasite genotype, host genotype and maternal environment on different stages of bacterial infection in Daphnia magna. Proc. R. Soc. B 279, 3176–3183. ( 10.1098/rspb.2012.0509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decaestecker E, Vergote A, Ebert D, De Meester L. 2003. Evidence for strong host clone–parasite species interactions in the Daphnia microparasite system. Evolution 57, 784–792. ( 10.1111/j.0014-3820.2003.tb00290.x) [DOI] [PubMed] [Google Scholar]
- 20.Carius HJ, Little TJ, Ebert D. 2001. Genetic variation in a host–parasite association: potential for coevolution and frequency-dependent selection. Evolution 55, 1136–1145. ( 10.1111/j.0014-3820.2001.tb00633.x) [DOI] [PubMed] [Google Scholar]
- 21.Little TJ, Chadwick W, Watt K. 2008. Parasite variation and the evolution of virulence in a Daphnia–microparasite system. Parasitology 135, 303–308. ( 10.1017/S0031182007003939) [DOI] [PubMed] [Google Scholar]
- 22.Luijckx P, Ben-Ami F, Mouton L, Du Pasquier L, Ebert D. 2011. Cloning of the unculturable parasite Pasteuria ramosa and its Daphnia host reveals extreme genotype–genotype interactions. Ecol. Lett. 14, 125–131. ( 10.1111/j.1461-0248.2010.01561.x) [DOI] [PubMed] [Google Scholar]
- 23.Ebert D, Rainey P, Embley TM, Scholz D. 1996. Development, life cycle, ultrastructure and phylogenetic position of Pasteuria ramosa Metchnikoff 1888: rediscovery of an obligate endoparasite of Daphnia magna straus. Phil. Trans. R. Soc. Lond. B 351, 1689–1701. ( 10.1098/rstb.1996.0151) [DOI] [Google Scholar]
- 24.Klüttgen B, Dulmer U, Engels M, Ratte HT, Dülmer U, Klttitgen B, Ratre HT. 1994. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 28, 743–746. ( 10.1016/0043-1354(94)90157-0) [DOI] [Google Scholar]
- 25.De Roode JC, Read AF, Chan BHK, Mackinnon MJ. 2003. Rodent malaria parasites suffer from the presence of conspecific clones in three-clone Plasmodium chabaudi infections. Parasitology 127, 411–418. ( 10.1017/S0031182003004001) [DOI] [PubMed] [Google Scholar]
- 26.Grech K, Watt K, Read AF. 2006. Host–parasite interactions for virulence and resistance in a malaria model system. J. Evol. Biol. 19, 1620–1630. ( 10.1111/j.1420-9101.2006.01116.x) [DOI] [PubMed] [Google Scholar]
- 27.Bell AS, de Roode JC, Sim D, Read AF. 2006. Within-host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution 60, 1358–1371. ( 10.1111/j.0014-3820.2006.tb01215.x) [DOI] [PubMed] [Google Scholar]
- 28.Mooij WM, Hülsmann S, Vijverberg J, Veen A, Lammens EHRR. 2003. Modeling Daphnia population dynamics and demography under natural conditions. Hydrobiologia 491, 19–34. ( 10.1023/A:1024451512597) [DOI] [Google Scholar]
- 29.Johnson PTJ, Stanton DE, Preu ER, Forshay KJ, Carpenter SR. 2006. Dining on disease: how interactions between infection and environment affect predation risk. Ecology 87, 1973–1980. ( 10.1890/0012-9658(2006)87[1973:DODHIB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 30.Auld SK, Hall SR, Housley Ochs J, Sebastian M, Duffy MA. 2014. Predators and patterns of within-host growth can mediate both among-host competition and evolution of transmission potential of parasites. Am. Nat. 184, S77–S90. ( 10.1086/676927) [DOI] [PubMed] [Google Scholar]
- 31.Cáceres CE, Knight CJ, Hall SR. 2009. Predator-spreaders: predation can enhance parasite success in a planktonic host–parasite system. Ecology 90, 2850–2858. ( 10.1890/08-2154.1) [DOI] [PubMed] [Google Scholar]
- 32.Ebert D. 2008. Host–parasite coevolution: insights from the Daphnia–parasite model system. Curr. Opin. Microbiol. 11, 290–301. ( 10.1016/j.mib.2008.05.012) [DOI] [PubMed] [Google Scholar]
- 33.Anderson RM, May RM. 1979. Population biology of infectious diseases: part I. Nature 280, 361–367. ( 10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 34.Obrebski S. 1975. Parasite reproductive strategy and evolution of castration of hosts by parasites. Science 188, 1314–1316. ( 10.1126/science.1145198) [DOI] [PubMed] [Google Scholar]
- 35.O'Keefe KJ, Antonovics J. 2002. Playing by different rules: the evolution of virulence in sterilizing pathogens. Am. Nat. 159, 597–605. ( 10.1086/339990) [DOI] [PubMed] [Google Scholar]
- 36.Ebert D, Carius HJ, Little T, Decaestecker E. 2004. The evolution of virulence when parasites cause host castration and gigantism. Am. Nat. 164, S19–S32. ( 10.1086/424606) [DOI] [PubMed] [Google Scholar]
- 37.Allen DE, Little TJ. 2011. Identifying energy constraints to parasite resistance. J. Evol. Biol. 24, 224–229. ( 10.1111/j.1420-9101.2010.02152.x) [DOI] [PubMed] [Google Scholar]
- 38.Mitchell SE, Rogers ES, Little TJ, Read AF. 2005. Host–parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution 59, 70–80. ( 10.1111/j.0014-3820.2005.tb00895.x) [DOI] [PubMed] [Google Scholar]
- 39.Andras JP, Ebert D. 2013. A novel approach to parasite population genetics: experimental infection reveals geographic differentiation, recombination and host-mediated population structure in Pasteuria ramosa, a bacterial parasite of Daphnia. Mol. Ecol. 22, 972–986. ( 10.1111/mec.12159) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.