FIG. 1.
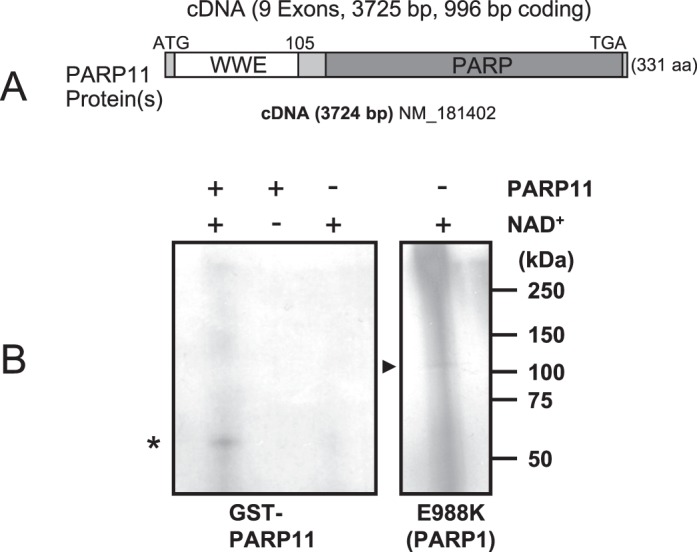
PARP11 is a mono(ADP-ribose) transferase. A) PARP11 protein contains two distinct domains, the WWE domain and the PARP domain. B) Automodification of PARP11 with monomeric ADP-ribose in the presence (+) or absence (−) of [32P]NAD+ as indicated by the presence of a defined band of the expected size of GST-PARP11 (*) and absence of an upward “smear” that would have indicated automodification with poly(ADP-ribose). As a positive control, an E988K mutant of the related protein PARP1 with known mono(ADP-ribosyl) transferase activity and low levels of poly(ADP-ribose) synthesis was used. It shows an equally sharp band of monomeric ADP-ribose (arrowhead) at the expected size of approximately 113 kDa (right), along with the typical upward smear indicating some residual poly(ADP-ribosyl)ation activity of the mutant. Note that overall acid-precipitable radioactivity was lower in PARP11, indicating a much lower specific activity of PARP11 compared to the PARP1 E988K mutant control.
