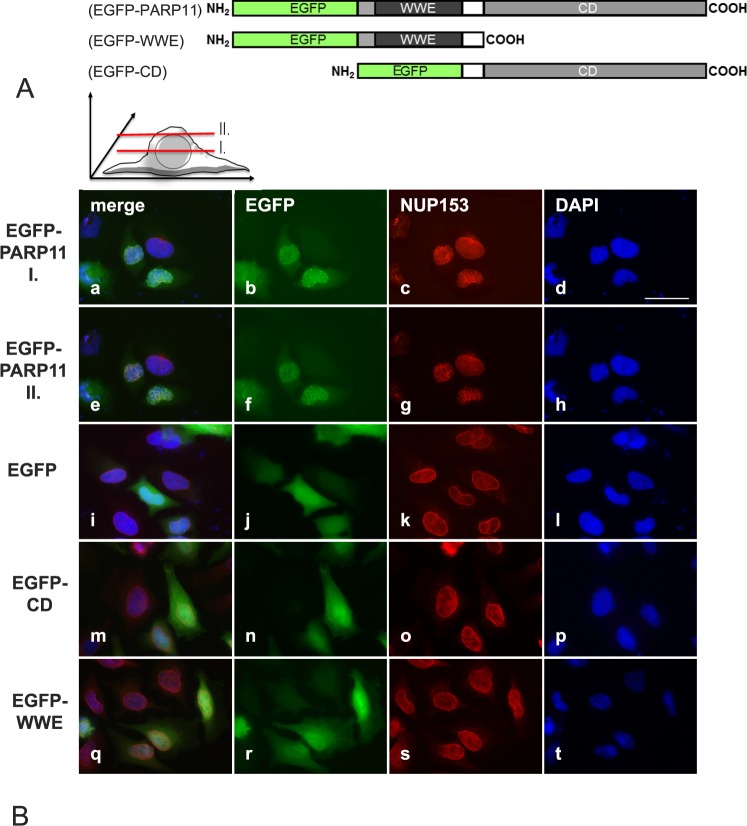
FIG. 2.
Overexpressed PARP11 preferentially localizes to the nuclear envelope in HeLa cells. A) PARP11-EGFP fusion protein structure. Eukaryotic expression vectors were generated that express fusion proteins consisting of either EGFP and the full-length PARP11 protein, EGFP fused to the PARP11 WWE domain only, or EGFP fused to the PARP domain only. B) Subcellular localization of the various EGFP-fusion proteins in transiently transfected HeLa cells. EGFP-PARP11 fusion were visualized with an EGFP-specific antibody (pseudo-colored green; b, f, j, n, and r). To localize the nuclear envelope, NUP153 was visualized by indirect immunofluorescence detections (pseudo-colored red; c, g, k, o, and s). DNA was counterstained with 4′,6-diamidine-2-phenylindole to visualize the nuclear area (pseudo-colored blue; d, h, l, p, and t). Merged images are shown in a, e, i, m, and q. EGFP-PARP11 fusion proteins comprising the full-length PARP11 protein preferentially localized to the nuclear envelope and colocalized with NUP153, a nuclear pore complex component. Images shown in rows EGFP-PARP11 I (a–d) and II (e–h) show the same cells imaged at slightly different focal planes, as illustrated in the schematic above images in B, to demonstrate colocalization of PARP11 with NUP153. Yellowish combined signals in the merged image (e) show colocalization. Images i–l reflect the signals observed for EGFP protein alone and show that the reporter EGFP alone did not exhibit any particular subcellular localization preference. Deletion of either the PARP11 WWE domain (EGFP-CD; m–p) or deletion of the catalytic domain (EGFP-WWE; q–t) completely abolished nuclear pore localization of PARP11. Bar = 40 μm.