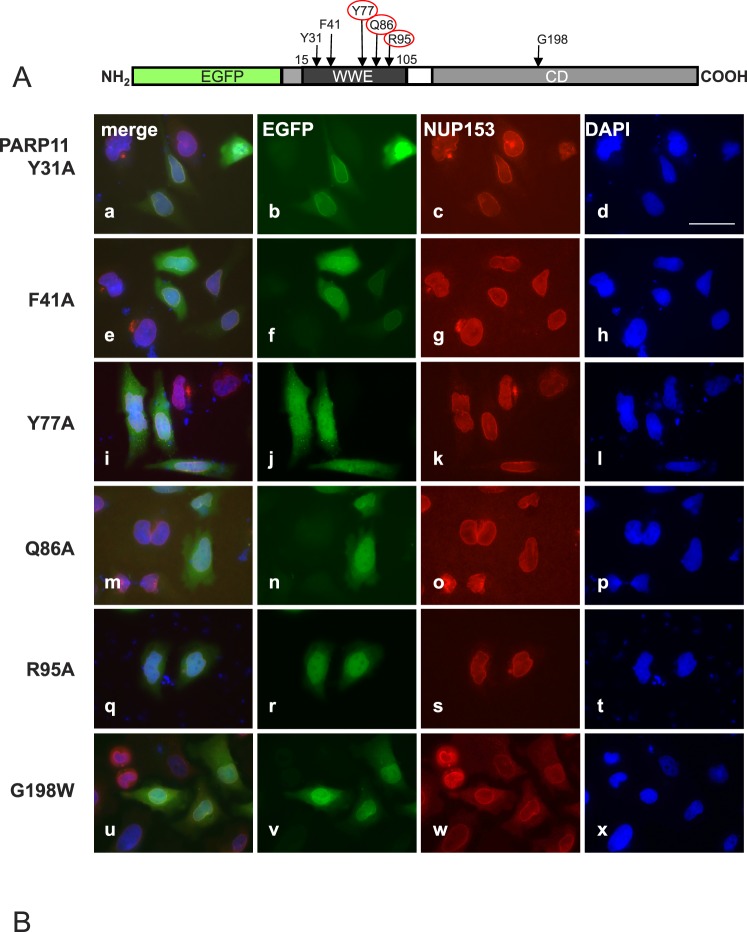
FIG. 3.
PARP11 nuclear envelope localization requires WWE domain amino acids (Y77, Q86, and R95) known to participate in ADP-ribose binding. A) Amino acids Y31, F41, Y77, Q86, and R95 in the WWE domain of PARP11 are involved in the binding to ADP-ribose [29]. To investigate the relevance of those amino acids for PARP11 subcellular localization, they were replaced with alanine (A) in expression vectors encoding the EGFP-PARP11 fusion protein. Positions of the particular amino acid exchanges within the EGFP-PARP11 protein are indicated by arrows. B) HeLa cells were transiently transfected to express the EGFP-PARP11 variants indicated on the left. Colocalization of mutant proteins with the nuclear envelope was evaluated by costaining with NUP153. EGFP fluorescence is visualized in green (b, f, j, n, r, and v) and NUP153 in red (c, g, k, o, s, and w). DNA was counterstained with 4′,6-diamidine-2-phenylindole (blue; d, h, l, p, t, and x). Merged images are shown in a, e, i, m, q, and u. Mutation of amino acids Y31 (a–d) and F41 (e–h) to A did not affect the observed subcellular localization pattern of the fluorescence signals compared to the original PARP11 fusion protein (see Fig. 2). In contrast, amino acid exchanges Y77A (i–l), Q86A (m–p), and R95A (q–t) ablated colocalization with NUP153 in the nuclear envelope and instead led to a fluorescence pattern comparable to that observed for EGFP only (see Fig. 2), indicating that presence of those residues (and likely ADP-ribose binding) is important for the association of PARP11 with the nuclear envelope. Mutation of an amino acid likely important for enzymatic activity (G198W; u–x) did not change EGFP-PARP11 subcellular localization. Bar = 40 μm.