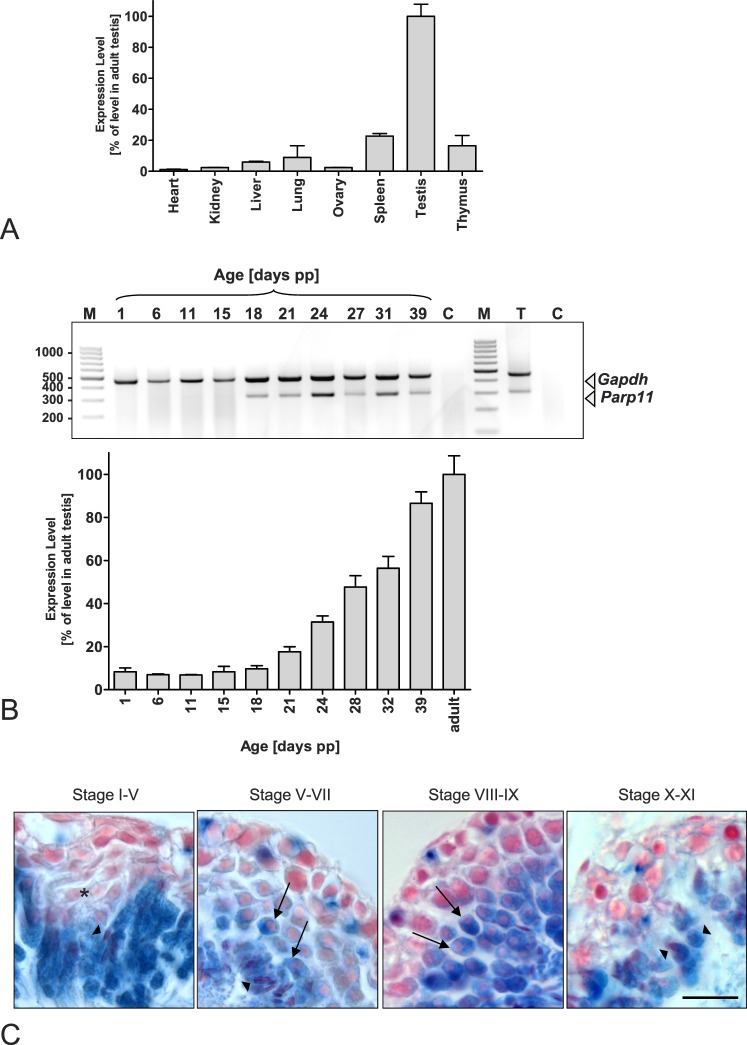
FIG. 4.
Parp11 is predominantly expressed during spermatid condensation in the testis. Qualitative and quantitative analyses of mRNA expression by end-point RT-PCR and quantitative PCR (qPCR) of total RNA extracted from tissues of adult wild-type mice demonstrate a preferential expression of Parp11 in the testis, particularly in spermatid stages just before the initiation of nuclear condensation. A) Parp11 mRNA was detectable at high levels in testis, whereas lower expression levels were detected in liver, lung, spleen, and thymus tissues. Messenger RNA levels in heart, kidney, and ovary were near the detection threshold. Gapdh was amplified as an internal control, and qPCR analysis of wild-type tissues is shown. Data are presented as the mean ± SD (n = 3 samples). B) RT-PCR products of RNA isolated from murine testes collected from animals at the indicated time points after birth. Testicular Parp11 expression is detectable from Postnatal Day 18 to adulthood using qPCR (top) and end-point RT-PCR (bottom) analyses. Quantitative PCR data are presented as the mean ± SD (n = 3 samples). C) Beta-galactosidase staining of testicular tissue from adult mice expressing the LacZ gene under the control of the Parp11 promoter. Blue cellular staining indicates Parp11 promoter activity. Early round spermatids lack blue staining (asterisk). Round spermatids from developmental steps 5–6 onward (arrows) and subsequent elongating and condensing spermatids (arrowheads) express beta-galactosidase, representing endogenous Parp11 promoter activity. Bar = 30 μm.