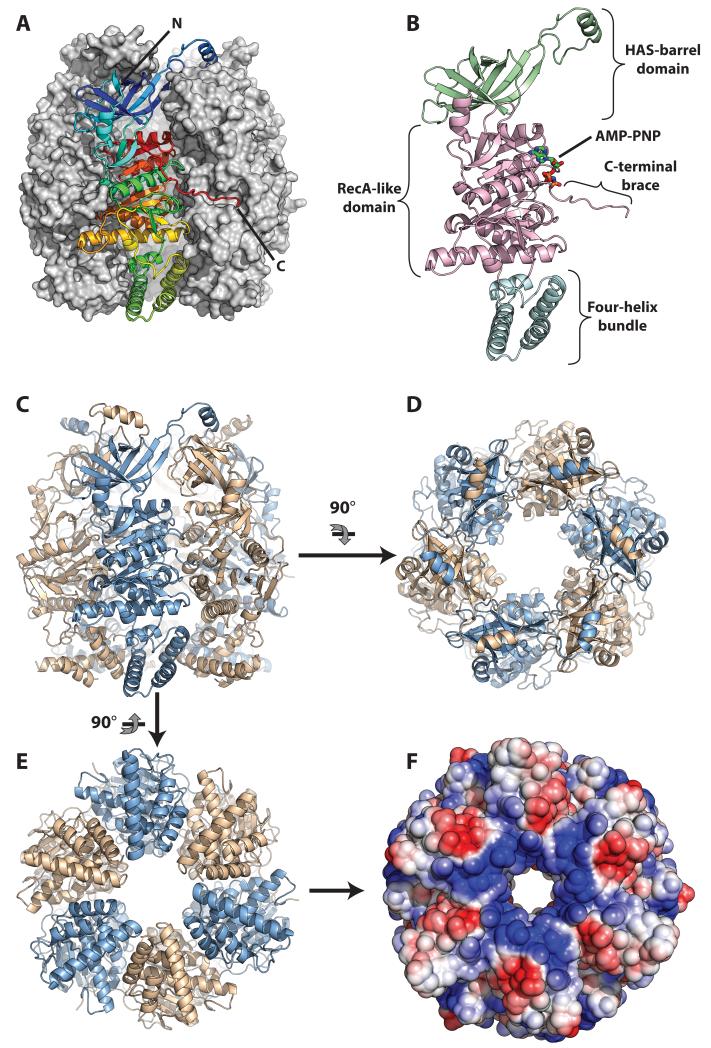
Figure 1. Architecture of the HerA helicase.
(A) Side view of the HerA hexamer generated by the three-fold crystallographic symmetry of the R3:H spacegroup. The foremost protomer is drawn as a ribbon and coloured from the N- (blue) to the C-end (red), while the other five protomers are shown as a grey surface. (B) Side view of a monomer of HerA, coloured by domain, with the HAS-barrel, ATPase and four-helix bundle domains in light-green, light magenta, and aquamarine respectively. The bound AMP-PNP is represented by coloured sticks. (C) Side view of the HerA hexamer with protomers coloured alternatively in light-blue and wheat. (D) Top-down view of the HerA hexamer. The HAS-barrel helical extensions embrace adjacent protomers. (E) Bottom-up view of the HerA hexamer. (F) Solvent-accessible surface representation of (E), coloured by electrostatic potential (+/− 3 kT, blue/red). The positively charged entrance to the HerA hexameric pore appears well suited to accommodate dsDNA. All figures generated using PyMOL (57); electrostatic surface calculated using APBS (58) and PDB id 2PQR (59).