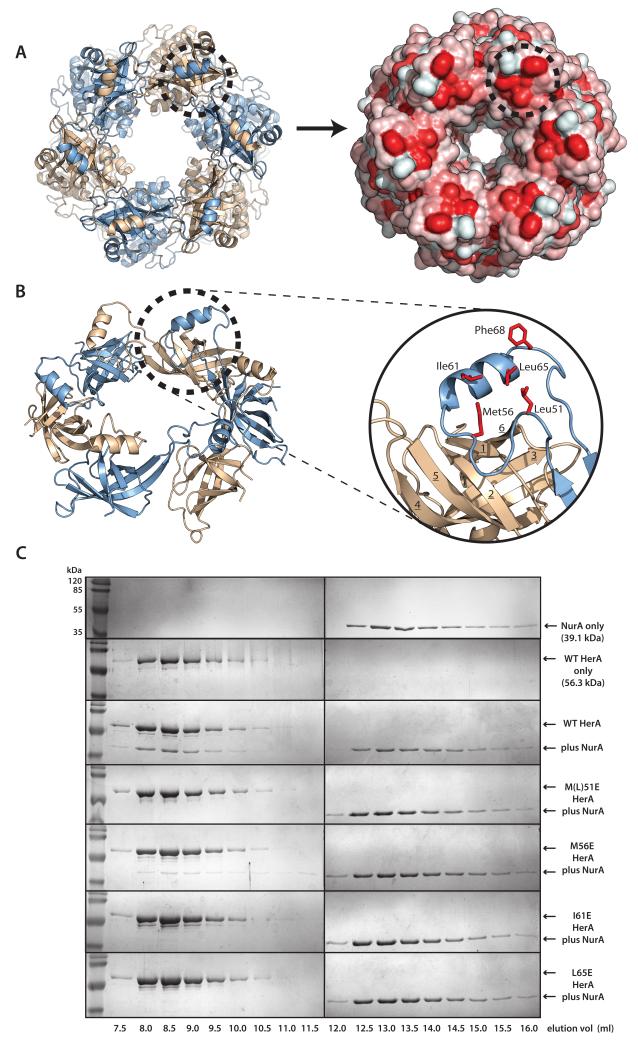
Figure 2. A conserved hydrophobic patch on the HerA HAS-barrel mediates NurA binding.
A helical extension from the N-terminal HAS- (HerA and ATP Synthase) barrel domain embraces the neighbouring protomer and forms an exposed hydrophobic surface that interacts with the NurA nuclease. (A) A hydrophobic patch on the HerA HAS-barrel (dotted circle). Left; Top-down view of the HerA hexamer (as shown in Figure 1D). Right; Surface representation, identifying exposed hydrophobic surfaces, coloured in red. (B) Cartoon representation of the toroidal arrangement of β-barrel domains crowning the HerA hexameric assembly (view tilted from (A) by 45°). A helical extension looping out between the 4th and 5th strands of the HAS-barrel domain is seen to embrace an adjacent barrel and form a hydrophobic patch (dotted circle). Inset; Close-up cartoon representation of the HAS-barrel helical extension (in blue), with surface-exposed hydrophobic residues (M51, M56, I61, L65 and F68) shown as red sticks. β-strands of the adjacent barrel (in wheat) are numbered 1 through to 6. Figures generated using PyMOL (57). (C) Characterisation of the NurA interaction surface of HerA. Gel filtration analysis of HerA-NurA complex formation, examining the interaction of NurA with either native HerA, or four individual HerA point mutations of residues exposed on the hydrophobic surface (M51E, M56E, I61E, L65E). Fractions eluted from the S200 HR 10/300 gel filtration column were resolved by SDS-PAGE. Chromatography UV traces for all elution profiles are displayed in Supplementary Fig. 3.