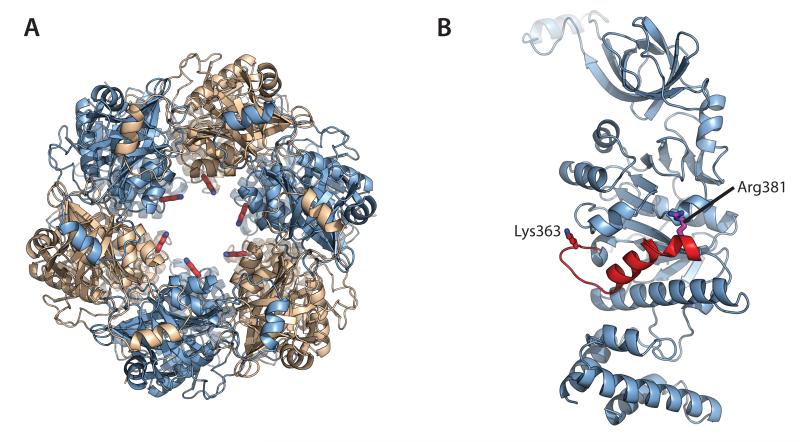
Figure 6. A conserved DNA-binding loop appears mechanically linked to the active site.
Each protomer of the HerA hexamer donates a conserved putative DNA-binding residue (K363) that protrudes into the DNA-binding channel from a flexible loop. (A) Left; Top-down view of the HerA hexamer, with Chain A protomers coloured light blue and Chain B protomers coloured wheat (as shown in Figure 1D). DNA-binding K363 residues (red sticks) are seen protruding into the central channel. (B) Cartoon representation of Chain A highlighting the putative DNA-binding loop, harbouring K363 (red stick). This lysine extends from the distorted helix (red) that also presents the key catalytic residue R381 (magenta stick) to the active site. All figures generated using PyMOL (57).