Figure 2.
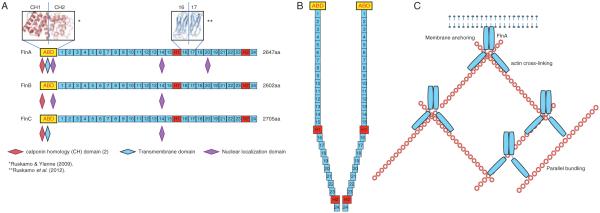
(A) FlnA is a 280 kDa protein consisting of 2647 amino acids with several different domains. There is an actin-binding domain (ABD) at the N-terminal end followed by 24 immunoglobulin (Ig)-like repeat domains, which are folded into β-sheets and together form a rod structure. The ABD of FlnA contains two calponin homology (CH) domains, with two actin-binding sites (ABS) in CH1 and one ABS in CH2. FlnA also contains a transmembrane domain in the ABD and three nuclear localization domains, located in repeats 1, 14, and 21. While the CH domains are conserved in FlnB and FlnC, FlnB does not contain the transmembrane domain and has only two nuclear localizations, located in repeats 1 and 14. FlnC does contain the transmembrane domain, but only has one nuclear localization in repeat 14. In all three, the 24 repeat domains following the ABD are interrupted by two hinge regions, the first (H1) is between repeats 15 and 16 and the second (H2) is between repeats 23 and 24. The repeat domains within FlnA have high sequence similarity and there is also high sequence similarity between the three filamin isoforms, with ~70% sequence similarity overall, and the lowest homology of only 45% at the hinge regions. (B) The molecules homodimerize by binding at repeat 24 and the hinge domains allow the protein to bend, so that when it dimerizes the structure is similar to a Y. (C) Dimerization allows FlnA to regulate the structure of the cell through actin binding in multiple ways. FlnA anchors the actin filamins at the membrane and can form them into perpendicular or parallel strands.
