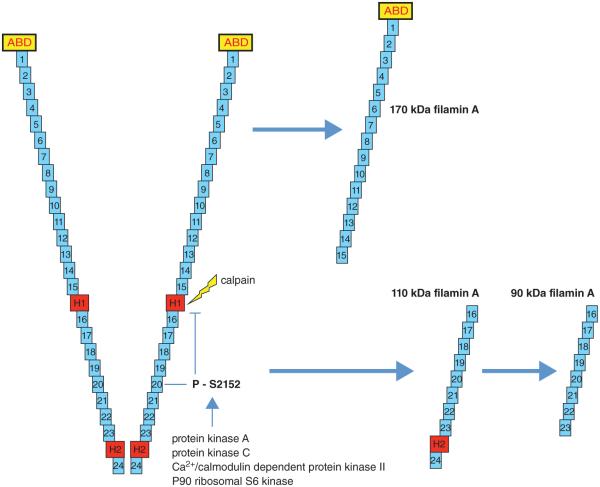
Figure 4.
FlnA is susceptible to proteolysis at the hinge regions. This cleavage can occur by calpain and caspase producing two proteins, a 170 kDa protein that includes the ABD and repeats 1–15 and a 110 kDa protein that is further cleaved to a 90 kDa protein that contains repeats 16–23. Susceptibility of FlnA to cleavage is regulated by its phosphorylation. Specific phosphorylation at S2152 in the 20th repeat is of particular importance because it is known to protect FlnA against cleavage. FlnA can be phosphorylated at S2152 by several different kinases including protein kinase A (cAMP-dependent protein kinase), protein kinase C, Ca2+/calmodulin-dependent protein kinase II, and p90 ribosomal S6 kinase. Other phosphorylation sites, including S2523, can influence location from the cytosol to the membrane and the interaction between FlnA surface receptors, but does not protect against cleavage.