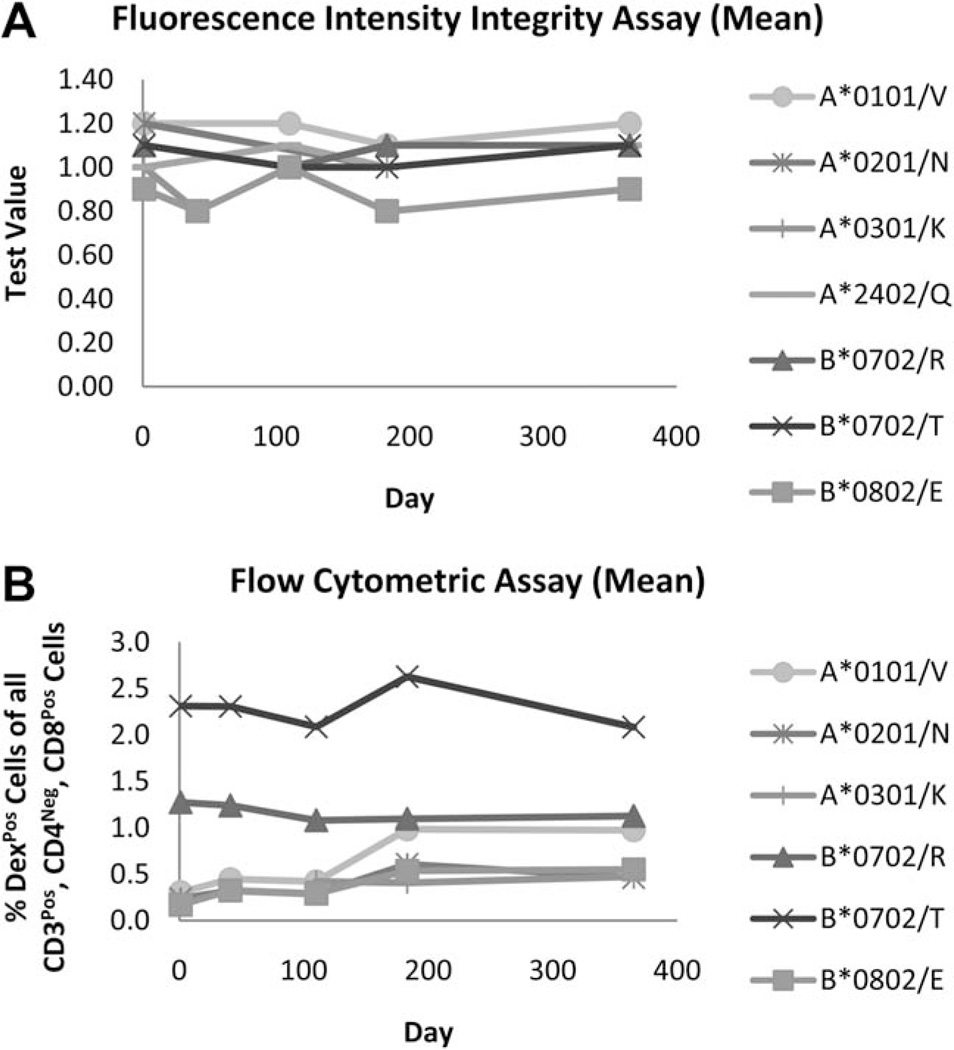
Fig. 4.
Dextramer reagents are stable for at least one year. A Fluorescence Intensity Integrity assay was performed to evaluate the stability characteristics of Dextramer reagents over time (A). Seven distinct PE-conjugated Dextramer reagents were synthesized and then assayed on days 1, 41, 110, 183, and 365 post-manufacturing for the ability to bind to an anti-β2-microglobulin-coated plate. Spectrophotometric measurements of PE emission were used to calculate a ‘Test Value’ (TV), (see Formulae 3–5 in the ‘Supporting Information and Methods’ section). The average TV for triplicate samples at each time point is plotted for Dextramer reagents of 7 different haplotypes and peptide specificities. These data demonstrate that for each reagent preparation tested, the amount of compositionally-intact Dextramer reagent is consistent over the course of the assay. The same lots of Dextramer reagents were also evaluated in a flow cytometric assay to demonstrate functional stability (B). These data demonstrate that for any given Dextramer reagent, the measured percentage of CASTs in PBMC samples is consistent over time, further indicating that Dextramer reagents are functionally stable for at least one year.