Figure 3. Small-RNA-driven transcriptional silencing of gene expression in C. elegans.
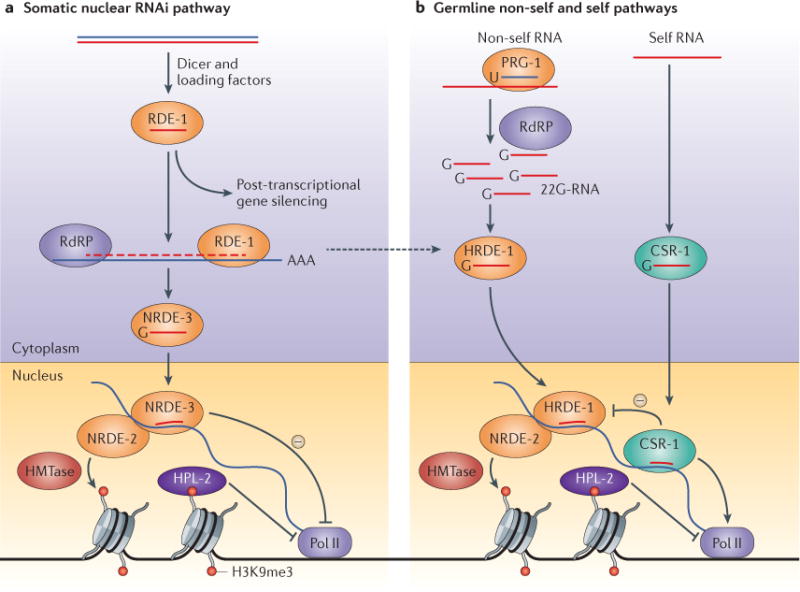
a | In Caenorhabditis elegans, exogenous double-stranded RNA (dsRNA) is processed into primary small interfering RNAs (siRNAs) that are loaded onto the Argonaute (AGO) protein RDE-1 and amplified by RNA-dependent RNA polymerases (RdRPs) to give rise to secondary siRNAs called 22G-RNAs. When loaded with 22G-RNAs, the somatic AGO protein NRDE-3 translocates to the nucleus, where it targets nascent RNA transcripts and silences corresponding genes, acting in concert with the silencing factor NRDE-2. Gene expression is halted by NRDE-2 during the elongation phase of transcription, and silencing involves histone H3 lysine 9 trimethylation (H3K9me3) and recruitment of the heterochromatin protein 1 (HP1)-like protein HPL-2. b | In the germ line, small-RNA-directed transcriptional silencing is mediated not by NRDE-3 but by a different AGO protein, HRDE-1, which also acts through NRDE-2, H3K9 methylation and HPL-2. HRDE-1 receives 22G-RNA inputs both from the pathway that responds to exogenous dsRNA and from the PIWI-interacting RNA (piRNA), or 21U-RNA, pathway that scans the transcriptome for foreign RNAs. 21U-RNA-programmed PRG-1 promotes the RdRP-dependent generation of 22G-RNAs, which are loaded onto HRDE-1. In both cases, HRDE-1 maintains a persistent, transgenerational memory of silenced genes in the germ line. Meanwhile, another AGO protein called CSR-1 binds to 22G-RNAs that represent the full complement of endogenously expressed RNAs, and protects the corresponding loci from possible silencing by HRDE-1. Thus, the 22G-RNAs bound by CSR-1 and HRDE-1 transmit a germline memory of ‘self’ and ‘non-self’ RNAs, respectively, to be appropriately licensed for expression or silenced. HMTase, histone methyltransferase; Pol II, RNA polymerase II.
