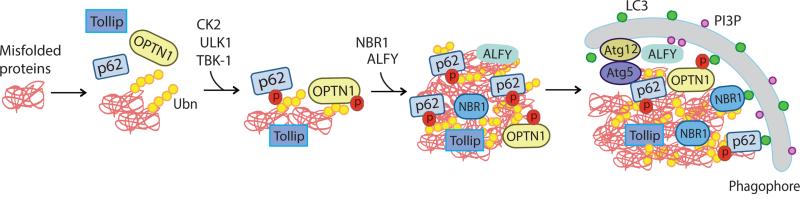
Figure 2. Regulation of aggrephagy: selective autophagy degrades protein aggregates via autophagy receptors.
Autophagy receptors, such as p62/SQSTM1, NBR1, OPTN1, ALFY and Tollip, mediate degradation of protein aggregates via selective autophagy by coupling autophagic cargos (ubiquitinated protein aggregates) and autophagy protein LC3. However, the binding affinity of certain autophagy receptors to either cargos (e.g. p62) or LC3 (e.g. OPTN1) is weak under normal condition. Phosphorylation of p62 by CK2, TBK1 and/or ULK1 enhances the affinity of p62 for ubiquitinated proteins (cargos), while phosphorylation of OPTN1 by TBK1 increases the binding of OPTN1 to autophagy modifier LC3. These modifications of p62 promote the selective degradation of protein aggregates by recruiting the autophagy machinery to the proximity of the aggregates.