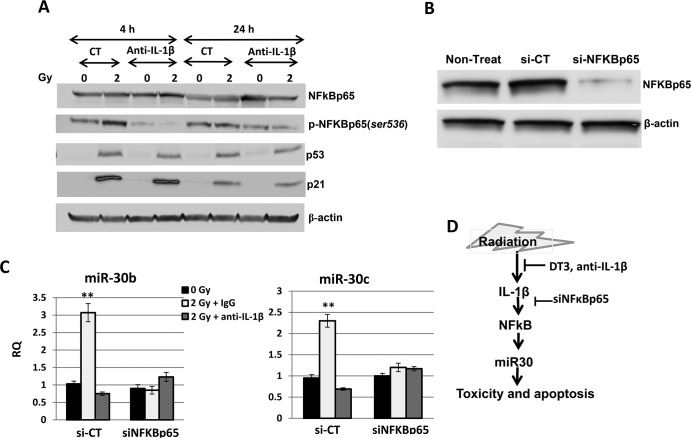
Fig 7. NFκB activation was responsible to radiation (and IL-1β)-induced miR-30 expression in CD34+ cells.
(A) CD34+ cells treated with control or anti-IL-1β antibody were collected at 4 h and 24 h after 0 or 2 Gy irradiation. p53, p21, NFκB expression and phosphorylation of NFκB (ser536) were examined by immunoblotting assay with β-actin serving as an internal control. Addition of anti-IL-1β antibody before radiation resulted in a significant inhibition of NFκB phosphorylation in these cells at 4 h and 24 h post-irradiation. (B) Western blots show NFκBp65 and β-actin (loading control) expression in non-transfected, control-siRNA-transfected, and NFκBp65-siRNA-transfected samples. (C) MiR-30b and miR-30c expression were validated in sham (0 Gy) or 2 Gy irradiated with or without anti-IL-1β antibody-treated and siNFκBp65 or control-siRNA transfected cells by quantitative real-time RT-PCR. RQ = relative quantitation; results from three independent experiments, **p<0.01; mean ± SD. 0 Gy vs. 2 Gy. (D) Schematic diagram of the role of DT3 or anti-IL-1β antibody in radioprotection. DT3 or anti-IL-1β antibody inhibited radiation-induced IL-1β production and reversed IL-1β-induced NFκB/miR-30 stress signaling.